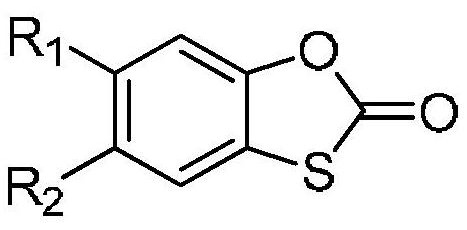
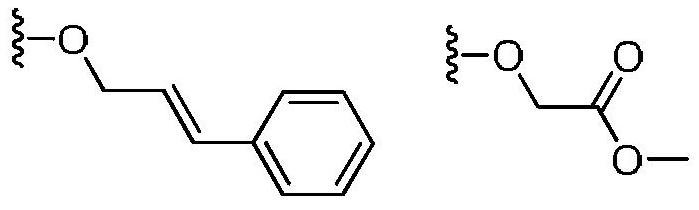
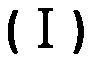Use of a 1,3-benzoxathiol-2-one compound in the preparation of monoamine oxidase inhibitors
A technology of benzoxathiol and ketone compounds, which is applied in the field of medicine and can solve problems such as differences in substrate selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the test of compound of the present invention to monoamine oxidase inhibitor activity
[0018] 1. Experimental reagents
[0019] Compounds of the present invention, MAOA (Active Motif, Cat.No.31502), MAOB (Active Motif, Cat.No.31503), Clogyline (Sigma, Cat.No.M3778), srigiline R(-)- deprenyl (Abcam, Cat.No.ab120604), 384-well plate (from Perkin Elmer, Cat.No.6007299)
[0020] 2. Experimental method
[0021] 1) Dissolve the compound of the present invention to 100 mM with 100% DMSO, add HEPES buffer in the multi-well plate, transfer the compound to the multi-well plate, and reduce the concentration of DMSO to 1%.
[0022] 2) Dissolve the enzyme and the substrate into the buffer solution respectively, transfer 10 μL of the substrate solution, start the reaction, let stand for 60 minutes, add 20 μL of luciferin, mix well, stand at room temperature for 20 minutes, measure and record the fluorescence signal The relative brightness of the compound was measure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com