A kind of amine alkoxychalcone compound and its preparation method and application
A technology of amine alkoxychalcone and amine alkoxybenzaldehyde, which is applied in the field of amine alkoxychalcone compounds and their preparation, can solve the problem of poor inhibitory activity of butyrylcholinesterase, inability to effectively prevent, To treat problems such as poor curative effect, achieve the effect of low cost, convenient operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
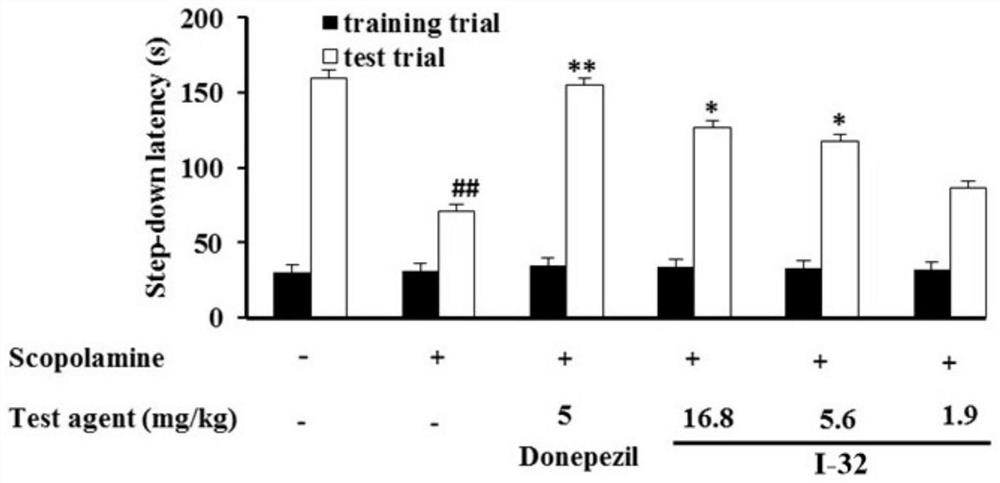
Image
Examples
Embodiment 1
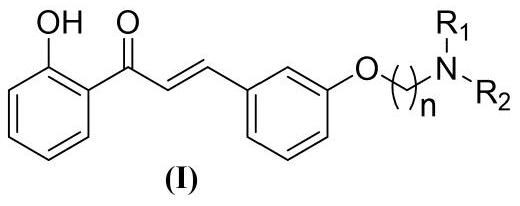
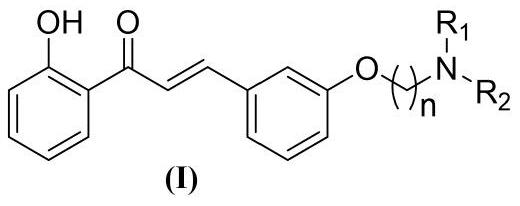
[0045] A kind of amine alkoxy chalcone compound, its general chemical structure formula is as shown in (I):
[0046]
[0047] Among them, n, R 1 , R 2 See Table 1 below for definitions.
[0048] Table 1 Amine alkoxy chalcone compound of the present invention
[0049]
[0050]
[0051]
[0052]
[0053] A preparation method of amine alkoxychalcone compound, comprising the following steps:
[0054] (1) Add 2.0mmol of the corresponding 3-hydroxybenzaldehyde (1), 3.0mmol of potassium carbonate, 6.0mmol of dibromide (2) and 15mL of acetonitrile into the reaction flask, heat to 65°C for 10 hours, and after the reaction, After conventional treatment, it was purified by silica gel column chromatography (eluent: petroleum ether: acetone = 50:1v / v) to obtain the corresponding alkoxybenzaldehyde (3);
[0055] (2) corresponding alkoxybenzaldehyde (3) 2.0mmol, secondary amine NR 1 R 2 (4) 2.5mmol and 3.0mmol potassium carbonate were added to a reaction flask containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com