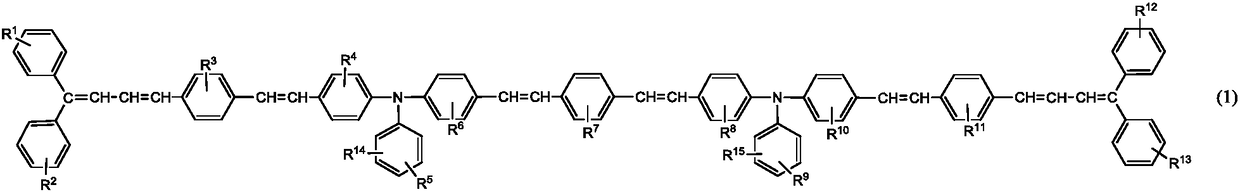
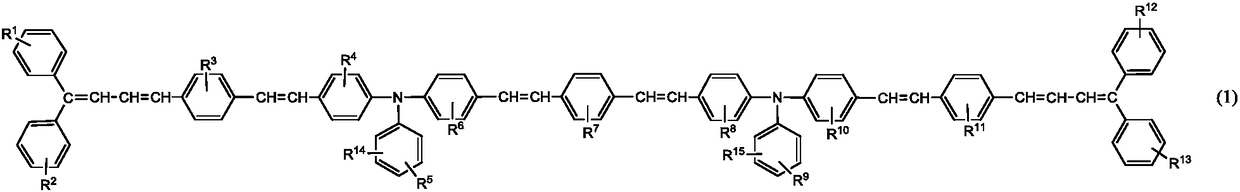
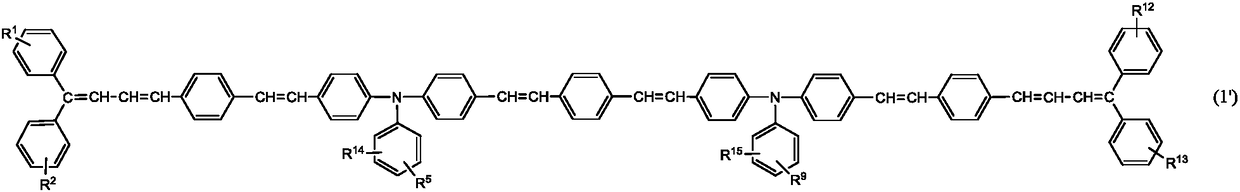
Triphenylamine derivative, charge transport material produced using same, and electrophotographic photoreceptor
一种电荷输送、衍生物的技术,应用在光学、电记录术、仪器等方向,能够解决电荷输送层载流子迁移率不是很高等问题,达到高载流子迁移率、工业上优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Synthesis of compound (1-1)
[0091] Under nitrogen atmosphere, add 8.38g (20mmol) of 4-(4'-(4",4"-diphenyl-1",3"-butadienyl)styryl) to 40ml of xylene Chlorobenzene, 2.4g (25mmol) of sodium tert-butoxide, 2.1g (20mmol) of p-benzylamine, 3.7mg (0.010mmol) of [PdCl (allyl)] 2 and 16 mg (0.040 mmol) of 1,1-diphenyl-2-(dicyclohexylphosphino)propene were heated at 100°C. After stirring for 3 hours, water was added, toluene was further added and the organic layer was extracted. After washing with water, it was concentrated and recrystallized from a solvent of toluene and methanol to obtain 8.0 g of a yellow solid. Wherein 7.5g was mixed with 40ml of xylene, further added 1.8g (19mmol) of sodium tert-butoxide, 2.6g (7.5mmol) of 1,4-bis(4-chlorostyryl)benzene, 2.7mg ( 0.008mmol) of [PdCl(allyl)] 2 and 12 mg (0.030 mmol) of 1,1-diphenyl-2-(dicyclohexylphosphino)propene were heated at 120°C. After stirring for 8 hours, water was added, toluene was further added and the organ...
Embodiment 2
[0096] Synthesis of compound (1-4)
[0097] Under nitrogen atmosphere, add 5.2g (12mmol) of 4-(4'-(4",4"-diphenyl-1",3"-butadienyl)styryl) to 40ml of xylene Chlorobenzene, 2.9g (30mmol) of sodium tert-butoxide, 1.1g (12mmol) of aniline, 4.4mg (0.012mmol) of [PdCl(allyl)] 2 and 19 mg (0.048 mmol) of 1,1-diphenyl-2-(dicyclohexylphosphino)propene were heated at 100°C. After stirring for 2 hours, 2.1 g (6.0 mmol) of 1,4-bis(4-chlorostyryl)benzene was added and further heated at 110°C. After stirring for 2 hours, water was added, toluene was further added and the organic layer was extracted. After washing with water, it was concentrated and recrystallized from a solvent of toluene and ethyl acetate to obtain 5.3 g of a yellow solid (compound 1-4). The yield was 72%. 1H NMR (CDCl 3 ): δ; 6.69-6.76 (m, 2H), 6.89-7.13 (m, 30H), 7.23-7.32 (m, 22H), 7.36-7.45 (m, 18H), 7.47 (s, 4H).
[0098] FD-MS ([M]+): Found m / z 1228.5647[C94H72N2]+ (calculated; 1228.5695, -3.95ppm)
[0099] m...
Embodiment 3
[0101] Synthesis of compound (1-7)
[0102] Under nitrogen atmosphere, add 6.3g (15mmol) of 4-(4'-(4",4"-diphenyl-1",3"-butadienyl)styryl) to 40ml of xylene Chlorobenzene, 1.8 g (19 mmol) of sodium tert-butoxide, 2.0 g (17 mmol) of 2,4-dimethylaniline, 5.5 mg (0.015 mmol) of [PdCl(allyl)] 2 and 23 mg (0.060 mmol) of 1,1-diphenyl-2-(dicyclohexylphosphino)propene were heated at 100°C. After stirring for 2 hours, water was added, toluene was further added and the organic layer was extracted. After washing with water, it was concentrated and recrystallized from a solvent of toluene and methanol to obtain 5.3 g of a yellow solid. Wherein 5.2g was mixed with 40ml of xylene, and 1.2g (13mmol) of sodium tert-butoxide, 1.8g (5.0mmol) of 1,4-bis(4-chlorostyryl)benzene, 1.8mg ( 0.005mmol) of [PdCl(allyl)] 2 and 7.8 mg (0.020 mmol) of 1,1-diphenyl-2-(dicyclohexylphosphino)propene were heated at 110°C. After stirring for 4 hours, water was added, toluene was further added and the orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com