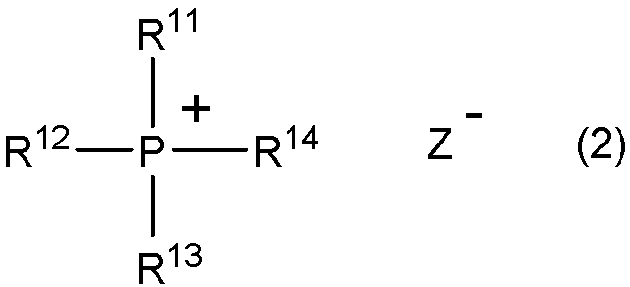Epoxy resin composition
A technology of epoxy resin and composition, which is applied in the direction of epoxy resin glue, epoxy resin coating, polishing composition, etc. It can solve the problems that it is difficult to simultaneously achieve excellent weather resistance of cured coating film, excellent adhesion of polycarbonate resin, etc. , to achieve the effect of excellent adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
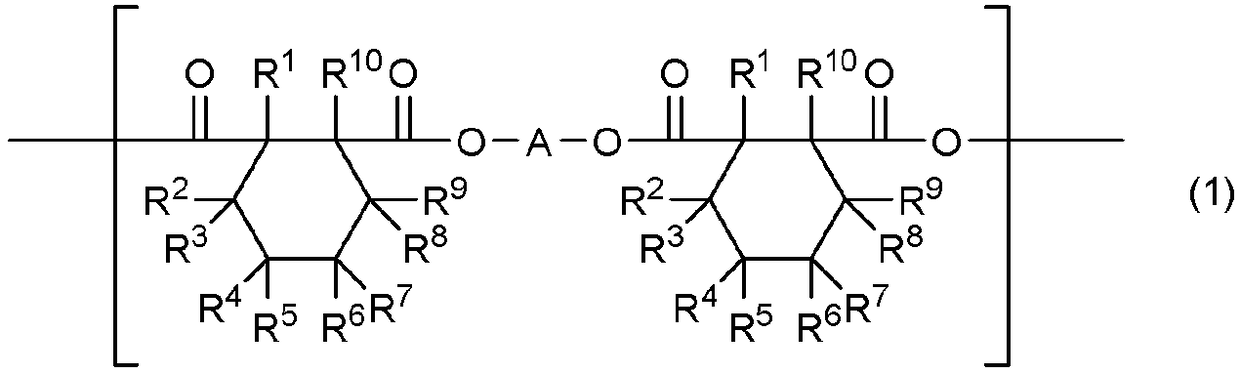
[0316] Specific examples include cyclohexanedicarboxylate anion, 3-methylcyclohexanedicarboxylate anion and 4-methylcyclohexanedicarboxylate anion.
[0317] Contain the organic anion (Z of the structure represented by formula (5) - ) Examples include organic sulfonic acid anions represented by formula (12)
[0318] R 28 -SO 3 - (12)
[0319] where R 28 means C 1-18 alkyl.
[0320] In formula (12) by R 28 represented by C 1-18 Alkyl is preferably straight chain or branched C 1-18 Alkyl, more preferably linear or branched C 1-12 alkyl. These alkyl groups include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, sec-pentyl, tert-amyl, Neopentyl, n-hexyl, isohexyl, sec-hexyl, tert-hexyl, neohexyl, n-heptyl, n-octyl, 2-ethylhexyl, n-nonyl, isononyl, n-decyl, isodecyl and n-decyl dialkyl. Among them, methyl is preferred.
[0321] Specific examples of the organic sulfonic acid anion represented by formula (12) include m...
Embodiment
[0386] Hereinafter, the present invention will be described in more detail with reference to examples. However, the present invention is not limited to these Examples. The properties described in Examples and Comparative Examples were each measured according to the following methods. For compounds not specified here, reagents are used. The compounds used in Production Examples 1 to 3, Examples 1 to 25, and Comparative Examples 1 to 19, their abbreviated names, and substrates are shown below.
[0387] Compound Abbreviation, Compound and Substrate Used
[0388] Anhydride
[0389] -HH: Rikacid HH, hexahydrophthalic anhydride (manufactured by Nippon Rika Co., Ltd.)
[0390] -MH-T: Rikacid MH-T, 4-methylhexahydrophthalic anhydride (manufactured by Nippon Rika Co., Ltd.)
[0391] alcohol
[0392] -HB: Rikabinol HB, hydrogenated bisphenol A (manufactured by Nippon Rika Co., Ltd.)
[0393] -27-DH: Decahydro-2,7-naphthalenediol (manufactured by Sugai Chemical Industry Co.,...
manufacture example 1
[0479] To a solution of 17.6 g of HB (73.0 mmol) in 15.0 g of butyl acetate, 22.5 g of HH (146.0 mmol, which is twice the amount of HB in mmol) was added, and the resulting product was incubated under a stream of nitrogen Stirring was carried out at 110° C. for 3 hours, thereby obtaining a solution of the dicarboxylic acid compound (HB / HH) in butyl acetate. To this dicarboxylic acid compound solution, 26.1 g of acetic anhydride (255.5 mmol, which is 3.5 times that of HB in terms of mmol) was added, and stirred at 100° C. for 1 hour under a nitrogen stream. Subsequently, the pressure inside the reactor was gradually reduced to 10.7kPa˜13.3kPa, and the solvent was added dropwise into the reactor and distilled off (butyl acetate was dropped and distilled off at a rate of 60 mL / hour) to allow the reaction to proceed for 5 hour, thereby obtaining polycarboxylic acid anhydride solution. Thereafter, the solution was diluted to 40% by weight with butyl acetate, and a solution of poly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com