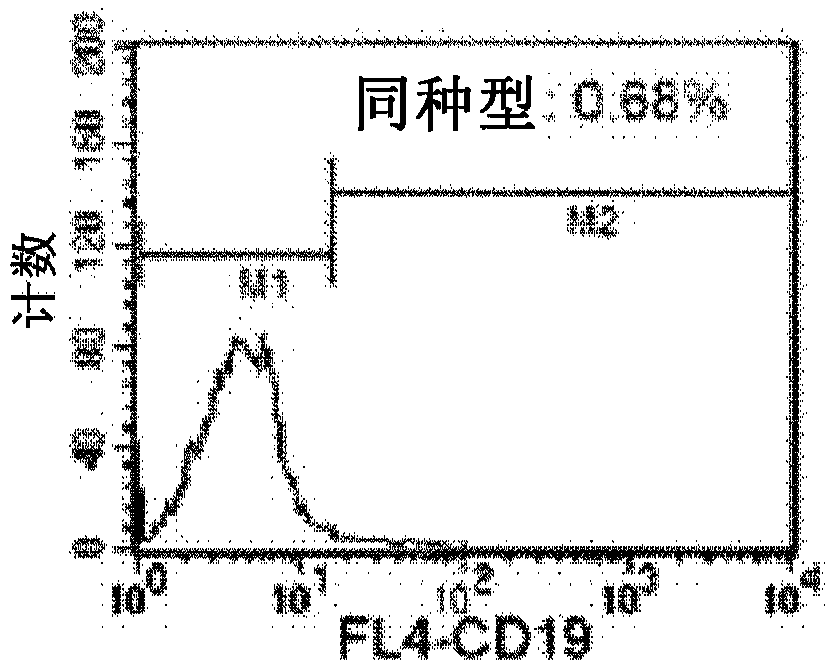
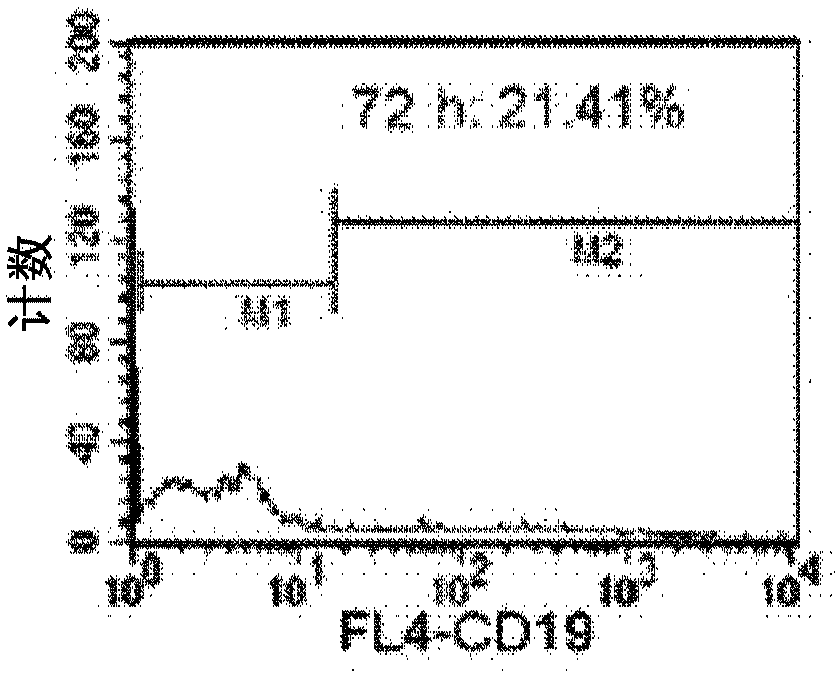
Methods for enhancing in vivo persistence and efficacy of exogenously administered t cells, genetically modified t cells and method and method of use
An exogenous, cellular technology, applied in receptor/cell surface antigen/cell surface determinant, chemical instruments and methods, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc. instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0080] Materials and methods
[0081] culture medium
[0082] T cells were cultured in T cell medium containing: X-VIVO™ 15 medium (Lonza, Basel, CH) supplemented with 5% fetal bovine serum (FBS) (Gibco, LAX, CA, USA), 100 U / mL Penicillin, 100 μg / mL streptomycin, 1.25 μg / mL amphotericin B, 2 mM L-glutamine (Gibco, CA, USA) and 100 U / mL hIL-2 (PerproTech, Rocky Hill, USA). T cells were activated using CD3- and CD28-specific magnetic beads (Invitrogen Life Technologies, Carlsbad, CA, USA) at 3 beads / cell. Raji and K562 cells were cultured in R10 medium containing: Roswell Park Memorial Institute (RPMI) 1640 (Hyclone, Logan, Utah, USA), supplemented with 10% fetal bovine serum (FBS), 100 U / mL penicillin, 100 μg / mL Streptomycin (Gibco, CA, USA) and 2 mM L-glutamine (Gibco, CA, USA). 293T cells were cultured in D10 medium containing: Dulbecco's modified Eagle's medium (Hyclone, UT, USA) supplemented with 10% FBS, 100 U / mL penicillin, 100 μg / mL streptomycin and 2 mM L-glutamine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com