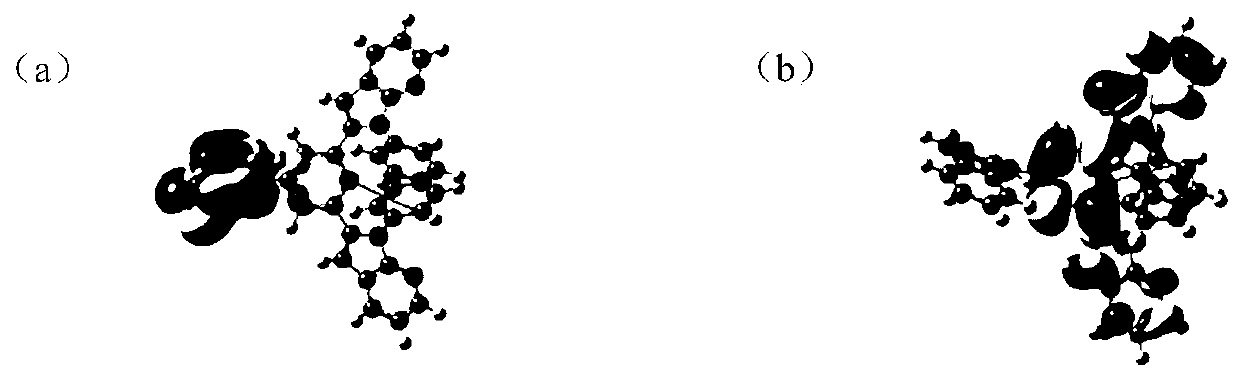A compound and preparation method, an organic light-emitting display device
A luminescent display and compound technology, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve problems such as low glass transition temperature, low molecular density, and low triplet energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0085] According to one embodiment of the present invention, the compound is selected from:
[0086]
[0087] Any one of them; Gaussian calculation results show that these structures have stronger electron-accepting capabilities, and the designed molecular triplet energy level is higher. In addition, these structures can also be easily synthesized or purchased directly.
[0088] Among them, D 1 and D 2 Each of them is independently selected from one or more of anilino group, aniline derivative substituent, carbazole derivative substituent, acridinyl group and acridine derivative substituent.
[0089] Among them, D 1 and D 2 Each unoccupied C atom on the phenyl group is substituted separately. D. 1 and D 2 Groups can be ortho, meta or para linked. When the position of the substituent is different, it may affect the conjugation effect, inductive effect, and configuration transformation of the molecule. According to one embodiment of the present invention, D 1 and D ...
Embodiment 1~16
[0197] Using density functional theory (DFT), for compound H1 to compound H16, using Gaussian 09 program package at B3LYP / 6-31G(d) calculation level, the distribution of molecular frontier orbitals was optimized and calculated.
[0198] figure 1 The orbital arrangement of compound H9 is shown, wherein, figure 1 (a) is the HOMO energy level distribution diagram of compound H9, figure 1 (b) is the LUMO energy level distribution diagram of compound H9. From figure 1 It can be clearly seen that the HOMO and LUMO of compound H9 are respectively arranged on different units, achieving a better separation between the donor and the donor, which is beneficial to the resonance between the donor and the donor group, Reduce energy difference △E between systems ST , thereby improving the intersystem crossing ability from singlet to triplet.
[0199] Among them, the relevant data of compounds H1-H16 are shown in Table 1. It can be seen from Table 1 that the triplet energy levels of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com