Osalmid crystal form II, and preparation method and application thereof
A technology of salivamol and crystal form, which is applied in the field of medicinal chemistry, can solve the problems such as the report of undiscovered salivamol crystal form, and achieve the effects of improving drug performance, crystal form with low hygroscopicity, and process improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
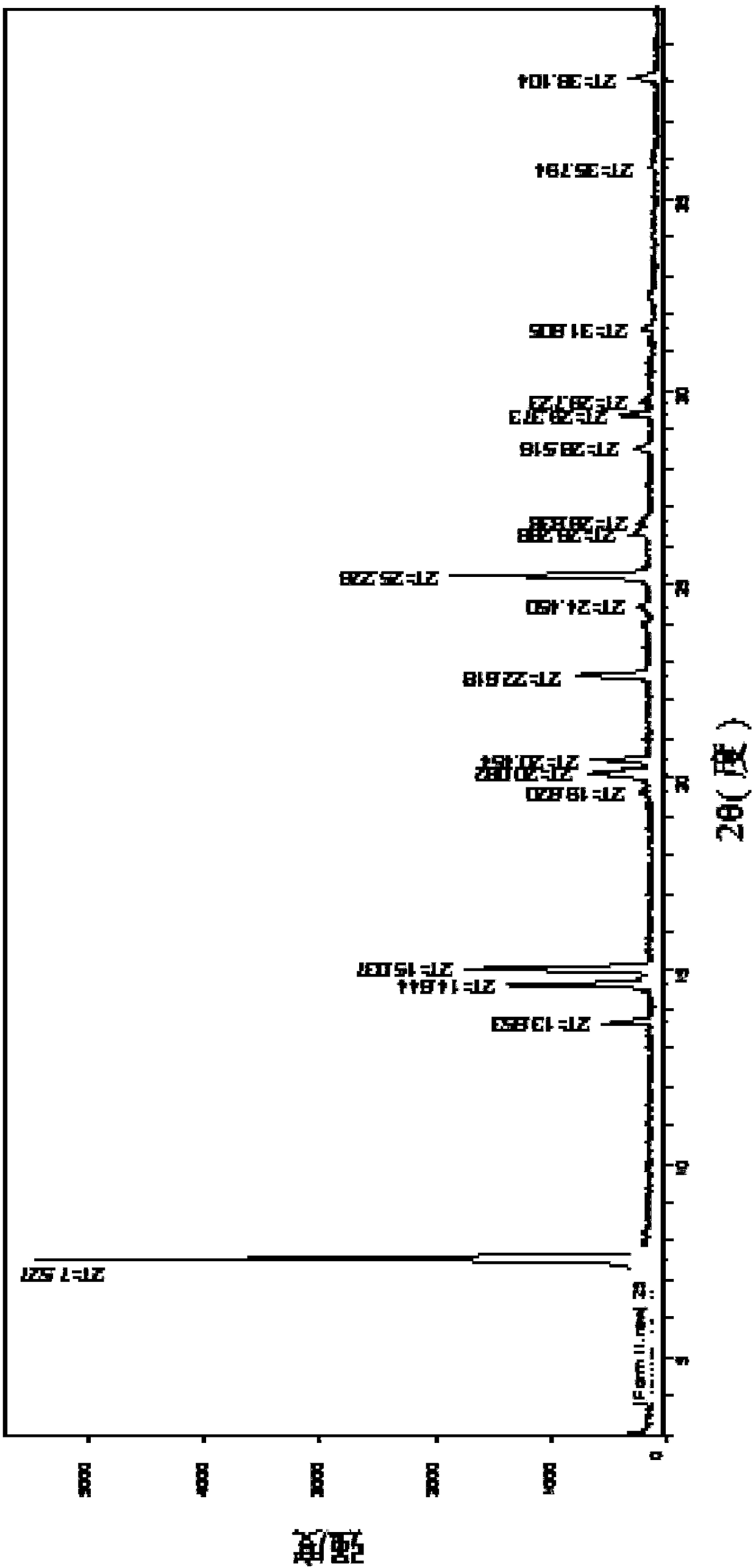
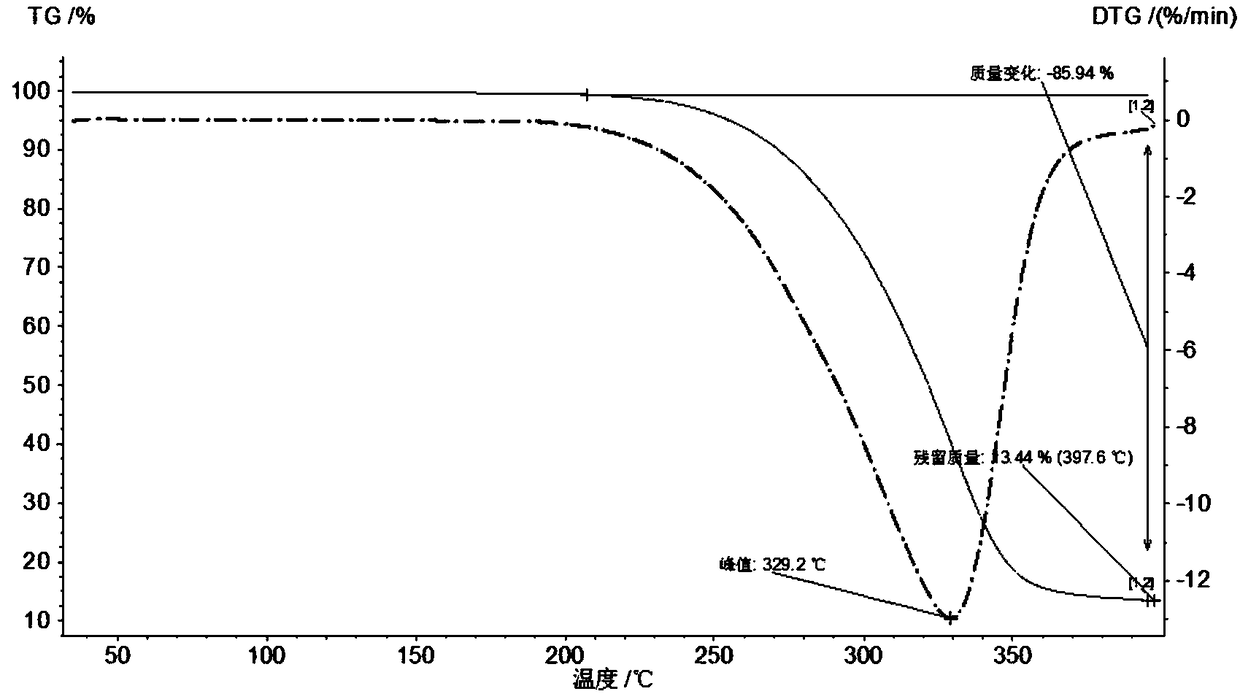
[0058] Example 1 Preparation of sulfanol crystal form II
Embodiment 1-1
[0060] Take about 3 g of sulfanol solid, add 400 ml of ethanol, mix and dissolve. Slowly volatilize to dryness at 25°C, and then collect the solid to obtain saliaminophen crystal form II, which is off-white crystals.
Embodiment 1-2
[0062] Take about 3 g of sulfanol solid, add 400 ml of ethanol and 400 ml of toluene, mix and dissolve. Slowly volatilize to dryness at 25°C, and then collect the solid to obtain saliaminophen crystal form II, which is off-white crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com