Clinical medicine laboratory instrument management system and standardized management method therefor
A technology of equipment management and management methods, which is applied in the field of medical clinical laboratory equipment management system and its standardized management, can solve the problems of easy contamination of operation cards, lower clinical and patient satisfaction, lack of record information, etc., to achieve reliable guarantee The effects of stable operation, convenient inspection and use, and improved management efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
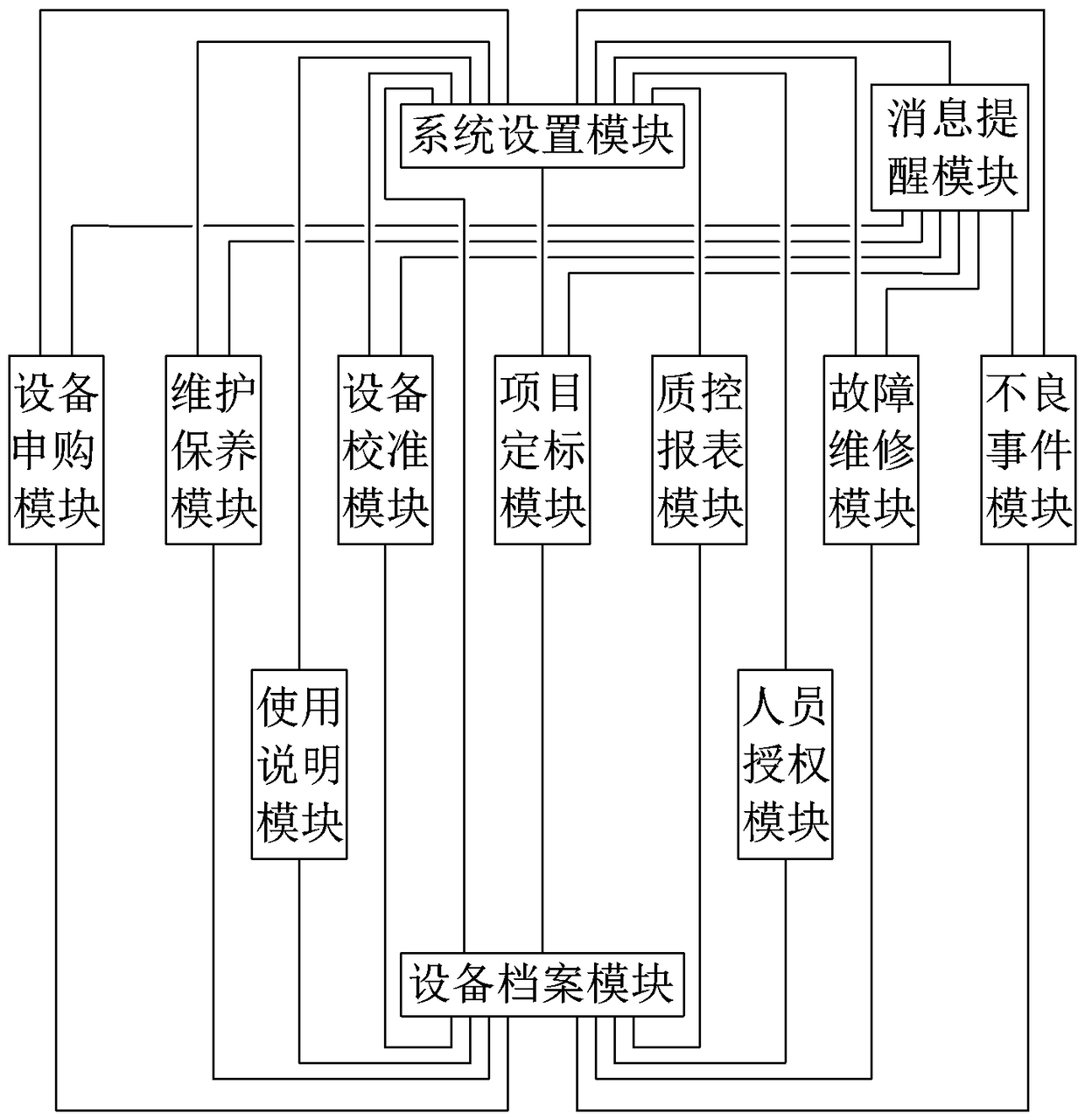
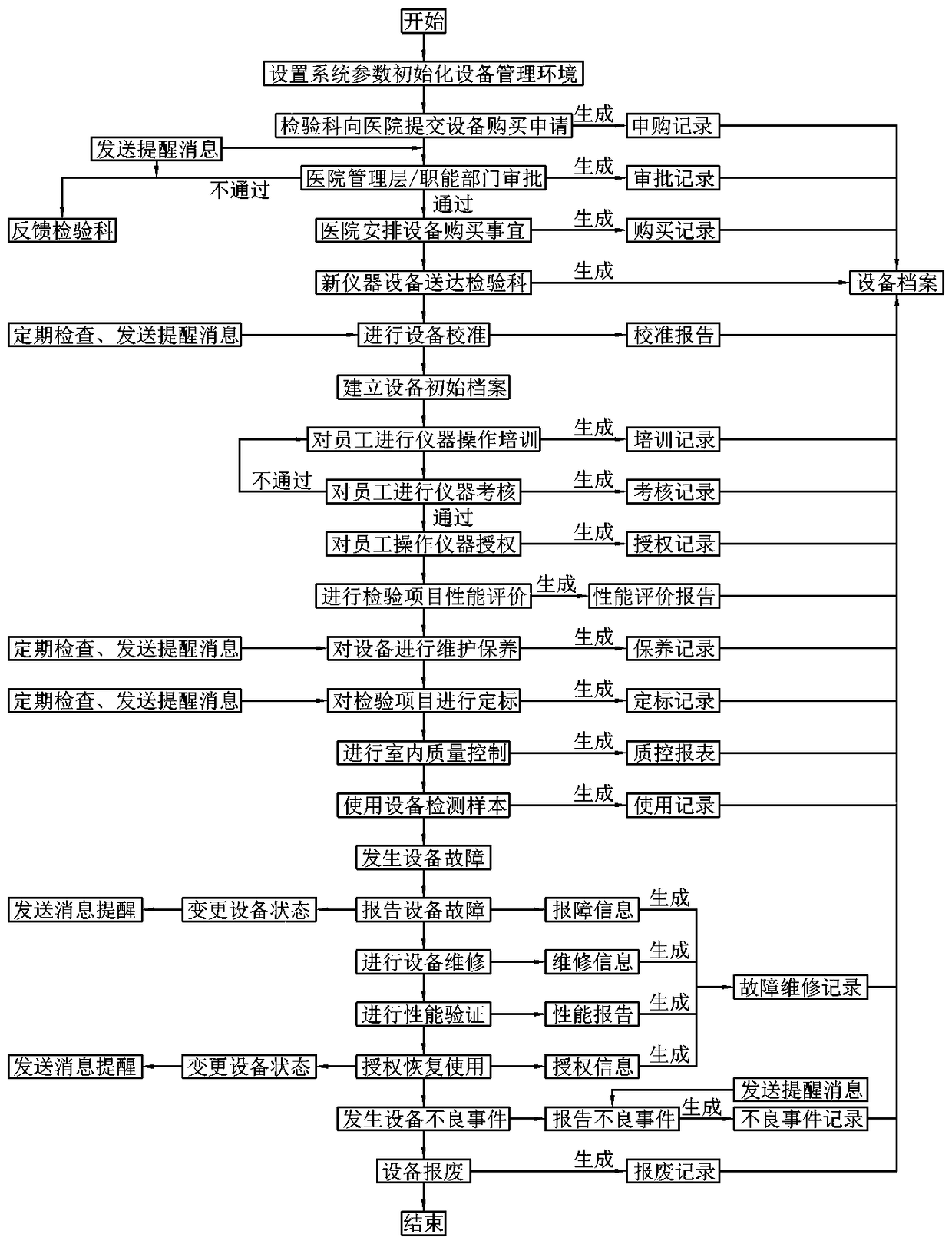
[0055] refer to figure 1 , a medical clinical laboratory equipment management system, the management system is composed of the following parts:
[0056] System setting module: used to set inspection equipment management departments (such as equipment department, laboratory department, clinical medical engineering center, etc.) and user accounts, roles and permissions, according to hospital and department management systems (such as hospital medical equipment procurement system, and management system, etc.) to set management processes such as equipment purchase and adverse event reporting, build a laboratory equipment management environment, and accurately control different departments and users to perform different operations;
[0057] Equipment purchase module: used for equipment purchase application, approval and management of purchase process records (such as bidding, bidding documents, etc.), the approval process automatically executes different approval processes accordin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com