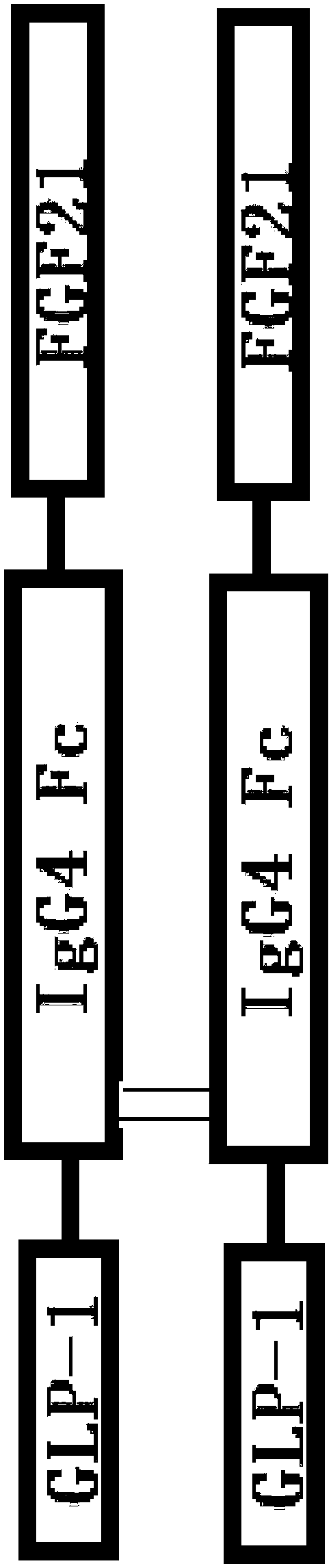
Double-target fusion protein containing immune globulin Fc portion
A fusion protein, FGF21 technology, applied in peptide/protein components, medical preparations containing active ingredients, fusion polypeptides, etc., can solve problems such as half-life extension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Embodiment 1: the construction of carrier
[0189] Using the method of molecular cloning, the vector of the fusion protein was constructed, and the fusion protein is shown in Table 1.
[0190] Table 1
[0191]
[0192]
[0193] The nucleotide sequence encoding dulaglutide and S1F1 fusion protein was obtained through chemical synthesis by entrusting GenScript Biotechnology Co., Ltd., and the vector encoding D1F1 fusion protein was synthesized by designing primers PCR template dulaglutide and S1F1 fusion protein The sequence was obtained to obtain fragments GLP-1 and Fc-FGF21, and the fragments were connected by SOE-PCR technology to obtain GLP-1-Fc-FGF21, which is the nucleotide sequence encoding the D1F1 mutant fusion protein.
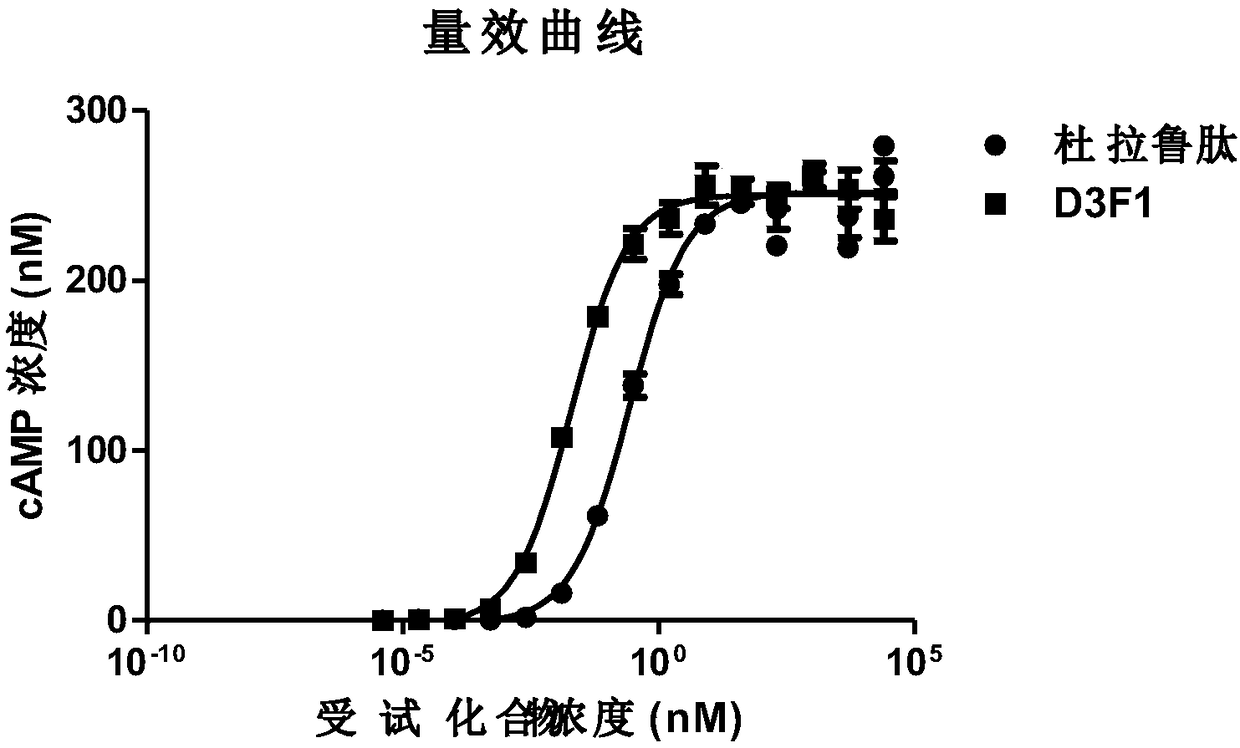
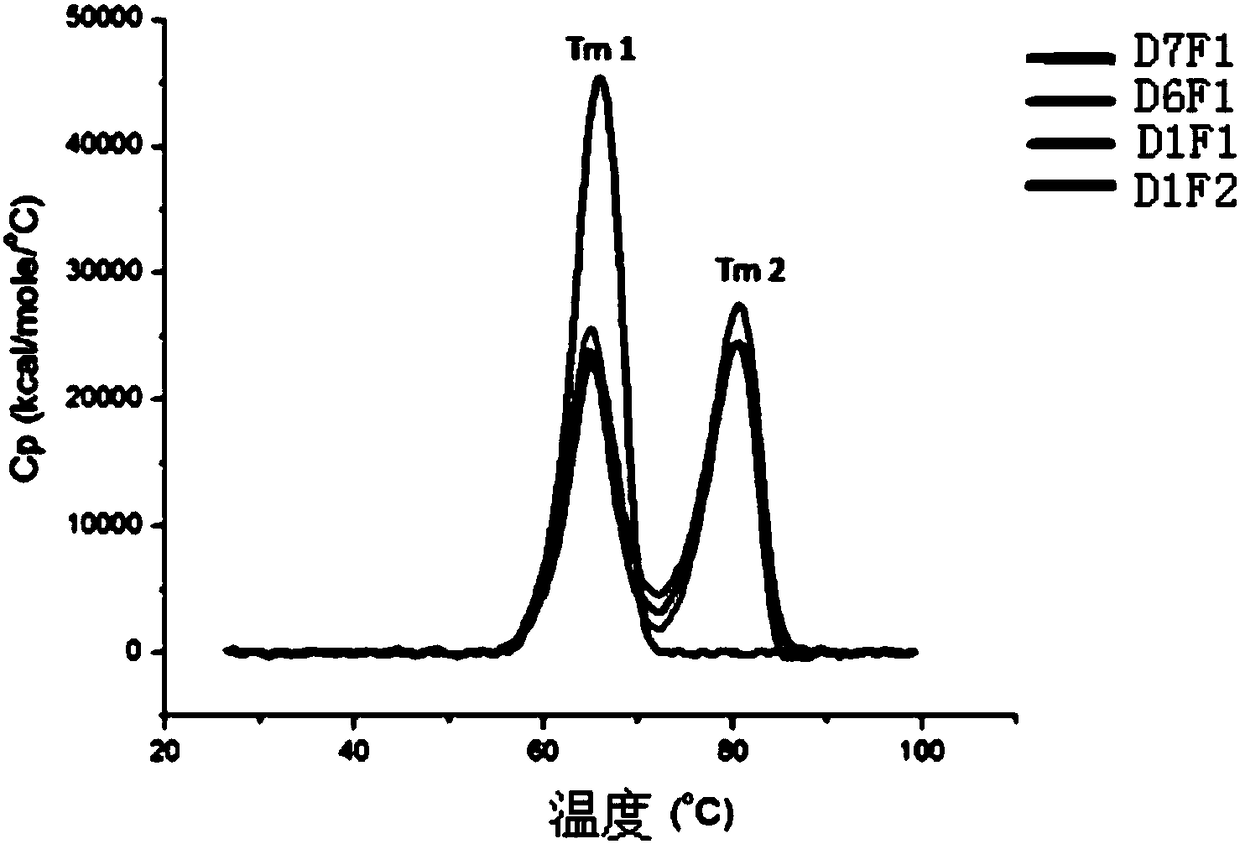
[0194] Nucleotide sequences encoding D1F2, D2F1, D3F1, D4F1, D5F1, D6F1 and D7F1 fusion proteins were obtained through chemical synthesis entrusted to Suzhou Jinweizhi Biotechnology Co., Ltd.
[0195] At 37°C, the nucleotide sequence and...
Embodiment 2
[0197] Example 2: Transfection of vector encoding dulaglutide and expression in cells
[0198] After recovery and culture of CHOK1SV GS-KO (Lonza) host cells with CD CHO medium (gibco), when the cell density was about 8x 10 5 Cells / mL were harvested for transfection. Transfected cells about 1x10 7 Cell, about 40 μg of vector, was transfected by electroporation (Bio-Rad, Gene pulSXcell). Cells were cultured in 20mL CD CHO medium after electric shock. On the second day of culture, the cells were collected by centrifugation at 200 g for 10 min, and resuspended in 20 mL of CD CHO medium with MSX (sigma) added to a final concentration of 50 μM. When the cell density is about 0.6x10 6 cell / mL, the obtained mixed clones were subcultured with CD CHO medium, and the subcultured cell density was about 0.2x10 6 cell / mL. When the cell survival rate was about 90%, the cell culture fluid was collected.
Embodiment 3
[0199] Example 3: Vectors encoding S1F1 and D1F1, D1F2, D2F1, D3F1, D4F1, D5F1, D6F1, D7F1 fusion proteins were transfected and expressed in cells
[0200] HEK293F host cells (Invitrogen, Freestyle 293F) were revived and cultured with 293Expression Medium medium (Invitrogen), when the cell density was about 1×10 6 Cells / mL were harvested for transfection. Transfected cells about 3x10 7 cell, the carrier is about 37.5μg, using FreeStyle TM MAX Reagent transfection kit was used. After transfection, the cells were cultured in 30 mL of 293Expression Medium. On the second day of cultivation, start to use geneticin (merck) to screen the transformants. According to the growth of the cells, replace the selection medium every 3 to 5 days. After about 14 days of selection, resistant clones can be seen, which can be carried out. Expand cultivation. The cell passage density is about 0.5x10 6 cell / mL, the obtained mixed clones were subcultured with 293Expression Medium medium. When ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com