Orally-taken meal supplementing medicament for treating osteoarthritis
A dietary supplement, osteoarthritis technology, applied in the fields of application, food science, food ingredients, etc., can solve the problems of increasing the pain of patients, not describing the use of magnesium and its compounds, associated with joint infections, etc., achieving low cost and high cost. Selective, good compliance effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] Example Magnesium and its compound Dietary Supplement Pharmacy Animal Experiment Verification of Curative Effects on Osteoarthritis
[0030] 1 Experimental materials and methods
[0031] 1.1 Drugs and reagents
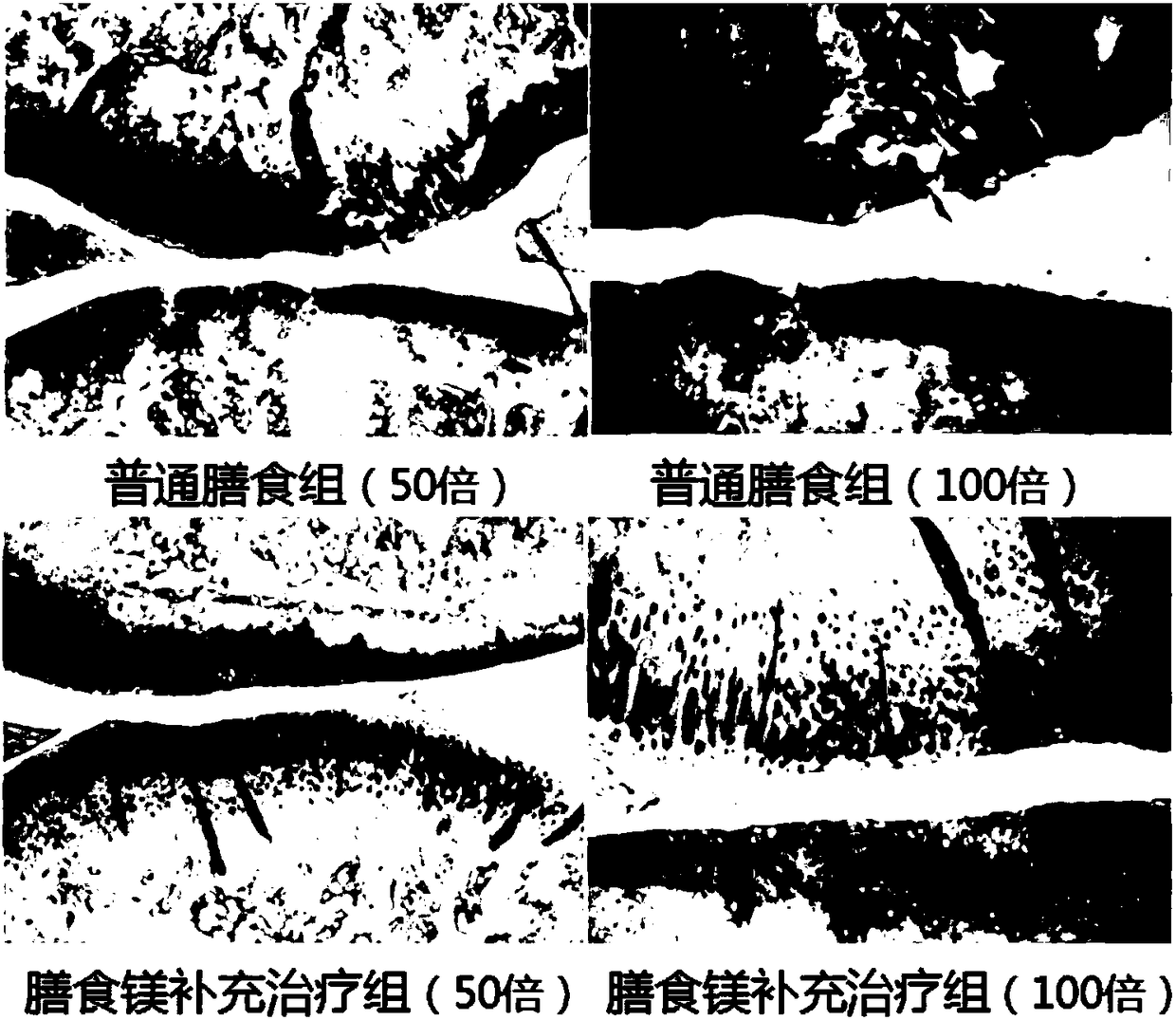
[0032] Ordinary rat feed (magnesium element content is 0.255g / kg)
[0033] Add the rat feed (magnesium element content is 2.55g / kg) of magnesia dietary supplement medicament
[0034] 1.2 Experimental animals
[0035] Adult male Sprague-Dawley (SD) rats (about 500g in weight)
[0036] 1.3 Experimental method
[0037] 1.3.1 Surgical Modeling
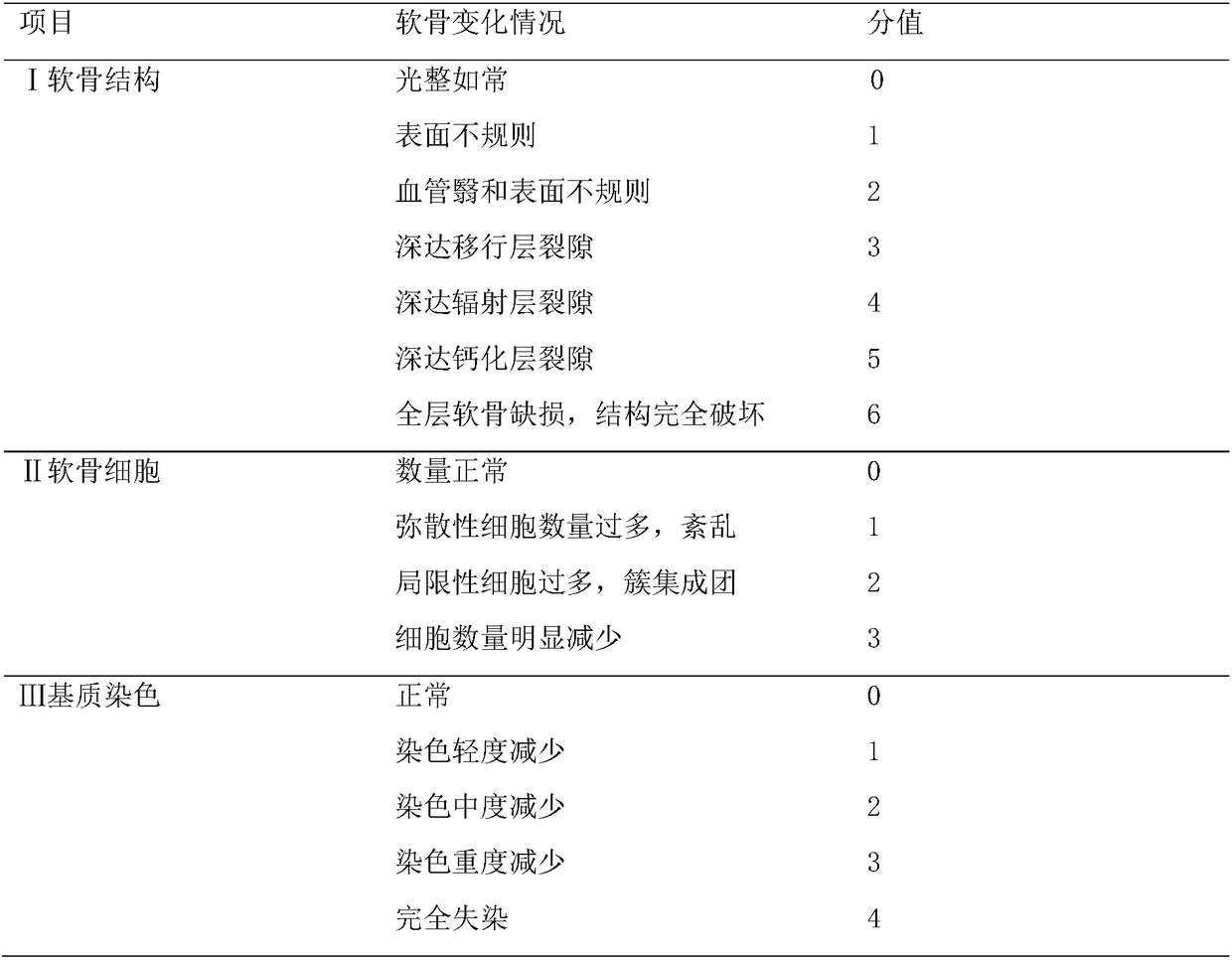
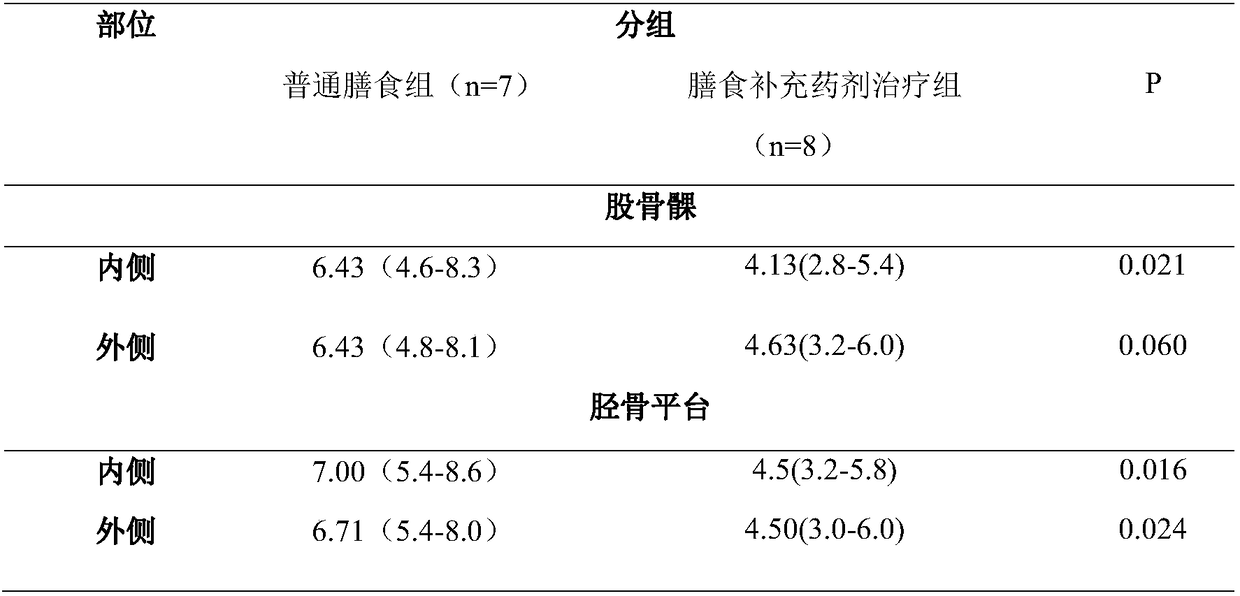
[0038] Knee OA model was constructed by right knee medial collateral ligament, anterior cruciate ligament transection and medial meniscectomy. This model has been validated to induce knee osteoarthritis and represents a good example of experimental degenerative osteoarthritis for pharmacological evaluation of tested drugs.
[0039] 1.3.2 Grouping and administration
[0040] After modeling, the rats were randomly divi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com