Lipoic acid transfusion preparation and preparing method thereof
A technology of lipoic acid and preparations, which can be used in anti-toxic agents, pharmaceutical formulations, drug delivery, etc., can solve problems such as lipoic acid exposure to light, unstable oxygen, high toxicity of ethylenediamine, and increased patient pain, so as to facilitate the safety of medication , Quality is easy to control, beneficial to the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] prescription:
[0020] Lipoic acid 3.0g;
[0022] Sodium hydroxide 0.005g;
[0023] Water for injection 1000ml.
[0024] Preparation Process:
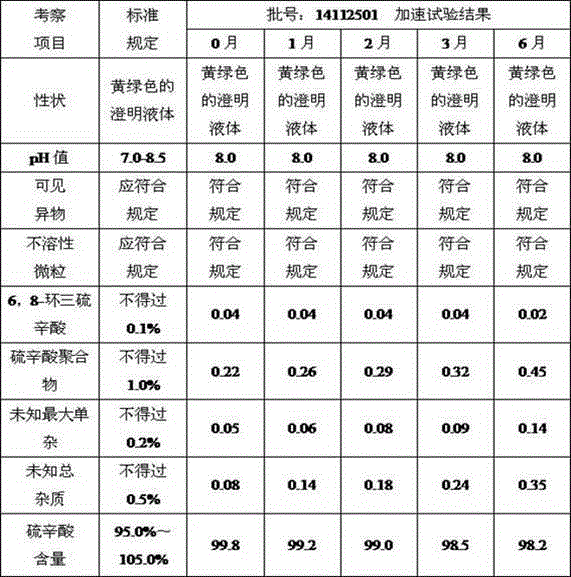
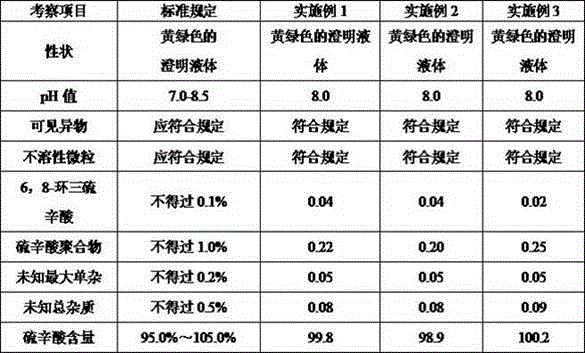
[0025] 3.0 g of lipoic acid and 0.5 g of sodium phosphate were alternately dropped into the water for injection filled with nitrogen and deoxygenated, and stirred to dissolve. Add 0.005g of sodium hydroxide to adjust the pH value to 7.7, and continue nitrogen protection; filter through 0.45 μm and 0.22 μm filter membranes successively, fill and seal under nitrogen protection; sterilize in a water bath at 121°C for 8-12 minutes, and the product is ready.
Embodiment 2
[0027] prescription:
[0028] Lipoic acid 6.0g;
[0030] Sodium hydroxide 0.05g;
[0031] Water for injection 1000ml.
[0032] Preparation Process:
[0033] Add lipoic acid and sodium phosphate alternately into the water for injection filled with nitrogen and deoxygenated, and stir to dissolve. Add an appropriate amount of sodium hydroxide to adjust the pH value to 8.0, and continue nitrogen protection. Filtered through 0.45μm and 0.22μm filter membranes successively, filled and sealed under nitrogen protection. Sterilize in a water bath at 121°C for 8-12 minutes.
Embodiment 3
[0035] prescription:
[0036] Lipoic acid 6g;
[0037] Sodium phosphate 0.75g;
[0038] Disodium hydrogen phosphate 0.25g;
[0039] Sodium hydroxide 0.01g;
[0040] Water for injection 1000ml.
[0041] Preparation Process:
[0042] Add lipoic acid, sodium phosphate, and disodium hydrogen phosphate alternately into the water for injection that has been filled with nitrogen and deoxygenated, and stir to dissolve. Add an appropriate amount of sodium hydroxide to adjust the pH value to 7.8, and continue nitrogen protection. Filtered through 0.45μm and 0.22μm filter membranes successively, filled and sealed under nitrogen protection. Sterilize at 121°C for 8-12 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com