Screening kit for metastatic screening of papillary thyroid microcarcinoma
A technology for papillary carcinoma and thyroid, which is applied in the determination/inspection of microorganisms, instruments, measuring devices, etc., can solve the problems of lack of transcriptomics, etc., and achieve the effect of avoiding over-treatment, rational use, and reducing psychological burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
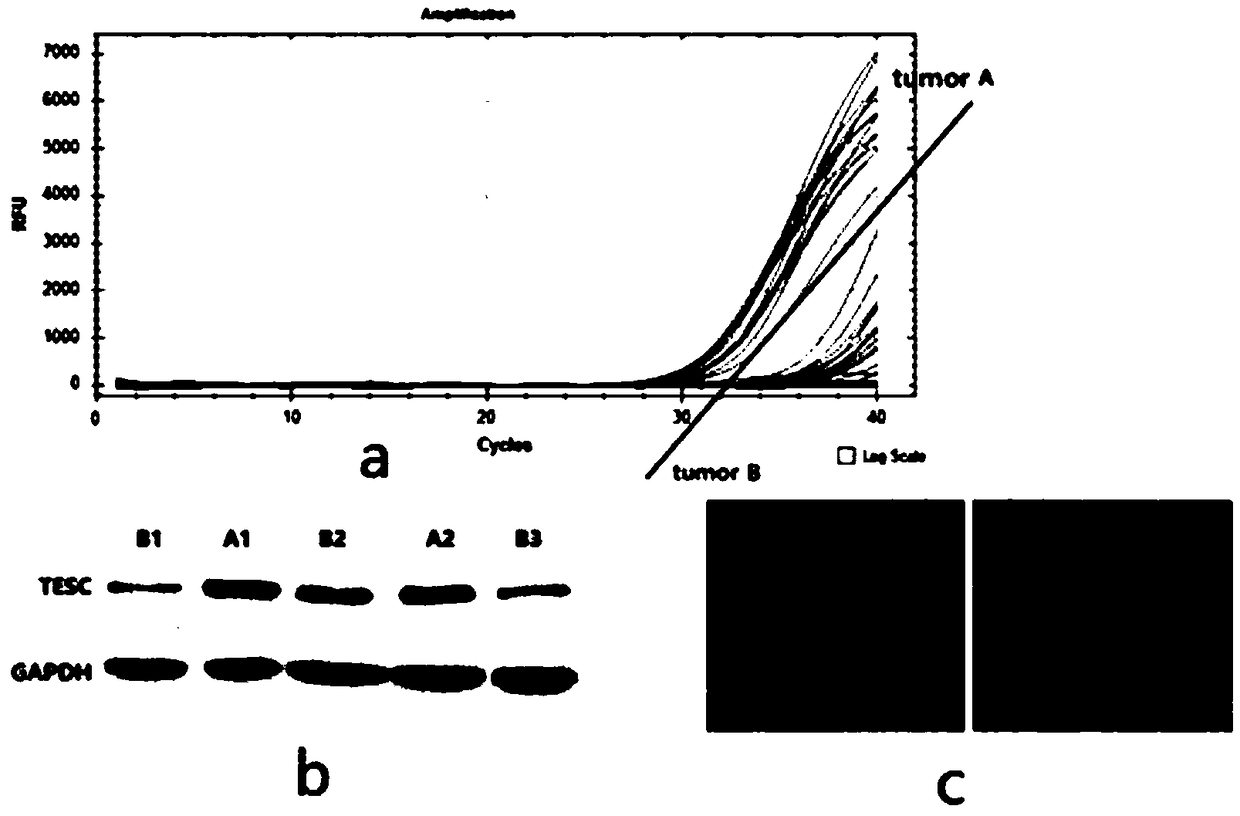
[0021] Example 1 The relationship between the expression level of TESC and the status of papillary thyroid microcarcinoma
[0022] 1. Pathology Collection
[0023] Collected PTMC cases from December 2016 to May 2018 in West China Hospital of Sichuan University, and signed informed consent for specimen collection before operation. Those who met the above conditions were routinely collected surgically resected pathological tissue samples. Within 15 minutes after excising the glandular lobe where the cancer focus is located, the cancer focus and normal tissue are collected and filled with liquid nitrogen, and the patient’s information is registered in detail to make a record. After a few months, a pathological test is performed to determine whether there is high lymph node metastasis or no metastasis. Strictly select and include The expression level of TESC was detected in the tumor tissues of the non-metastasis and high-metastasis groups, 35 cases without metastases and 26 cases...
Embodiment 2
[0048] Embodiment 2 The present invention detects the composition of the kit of TESC and using method thereof
[0049] 1. PCR detection kit
[0050] 1. The composition of the kit
[0051] Detection kit (50 copies):
[0052] components
volume
upstream primer
0.5ul (10μM)
downstream primer
0.5ul (10μM)
2×PCR Enzyme Mix
10 μL
dd water
to a total volume of 20 μL
[0053] The primer sequences are as follows:
[0054] TESC: Forward: TCAACCCCATCCGATCCAAA
[0055] Reverse: ATCTCAGCTTCTCCTTCCGG
[0056] 2. How to use the kit
[0057] Take out the tumor tissue of the sample to be tested, place it on ice for 5 minutes to soften it, pulverize it with a tissue homogenizer, weigh it, and add 1ml TRIZOL per 100mg tissue. Add 0.2ml of chloroform to every 1ml of TRIZOL reagent lysed sample, and cap the tube tightly. Shake the tube vigorously by hand for 15 seconds, and incubate at 15 to 30°C for 2 to 3 minutes. Centrifuge at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com