Application of blood serum sST2 in dilated cardiomyopathy prognosis
A dilated cardiomyopathy, pediatric technology, applied in the fields of treatment and prognosis, disease diagnosis, and biotechnology, can solve the problem of unknown predictive value of sST2 in children with dilated cardiomyopathy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
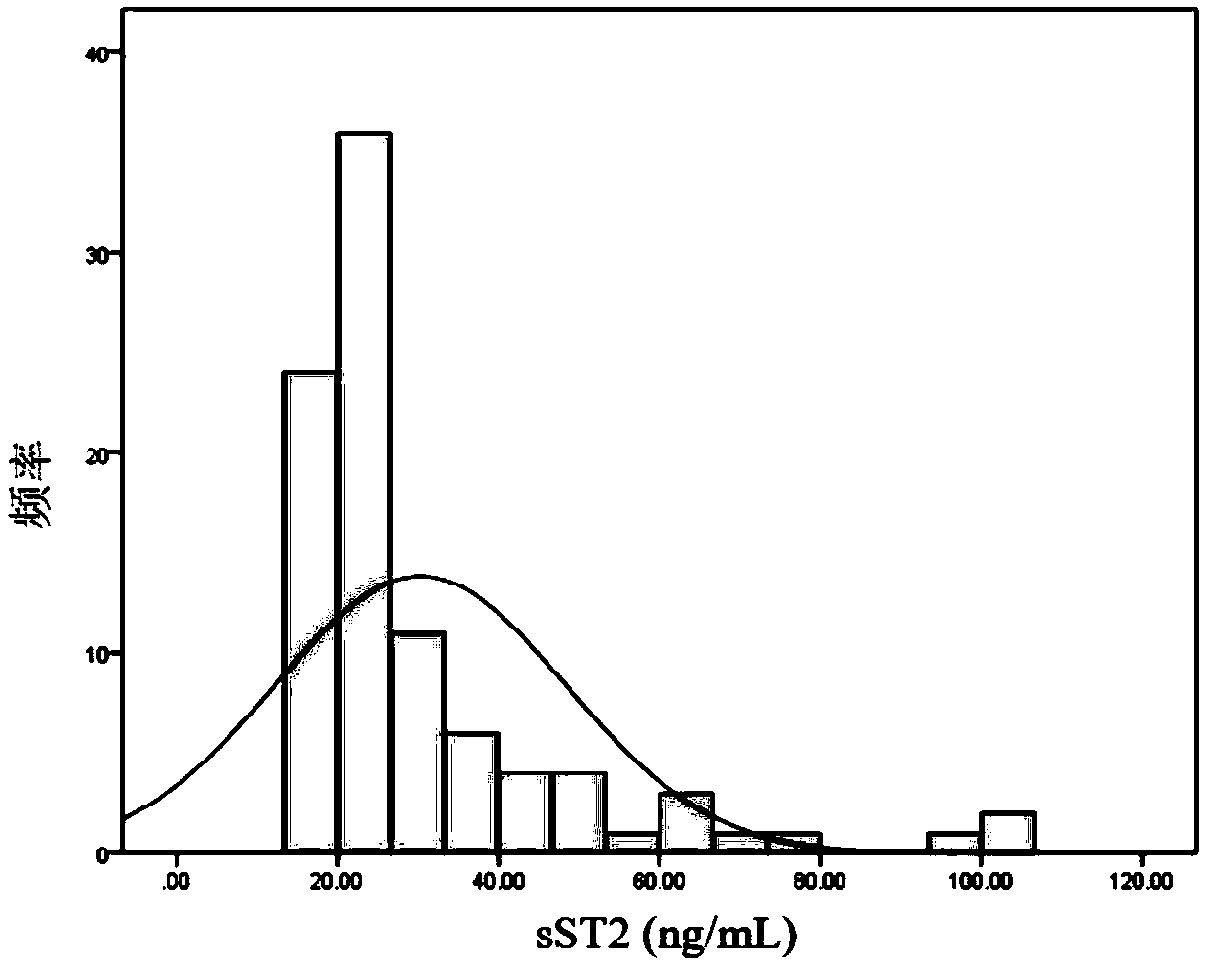
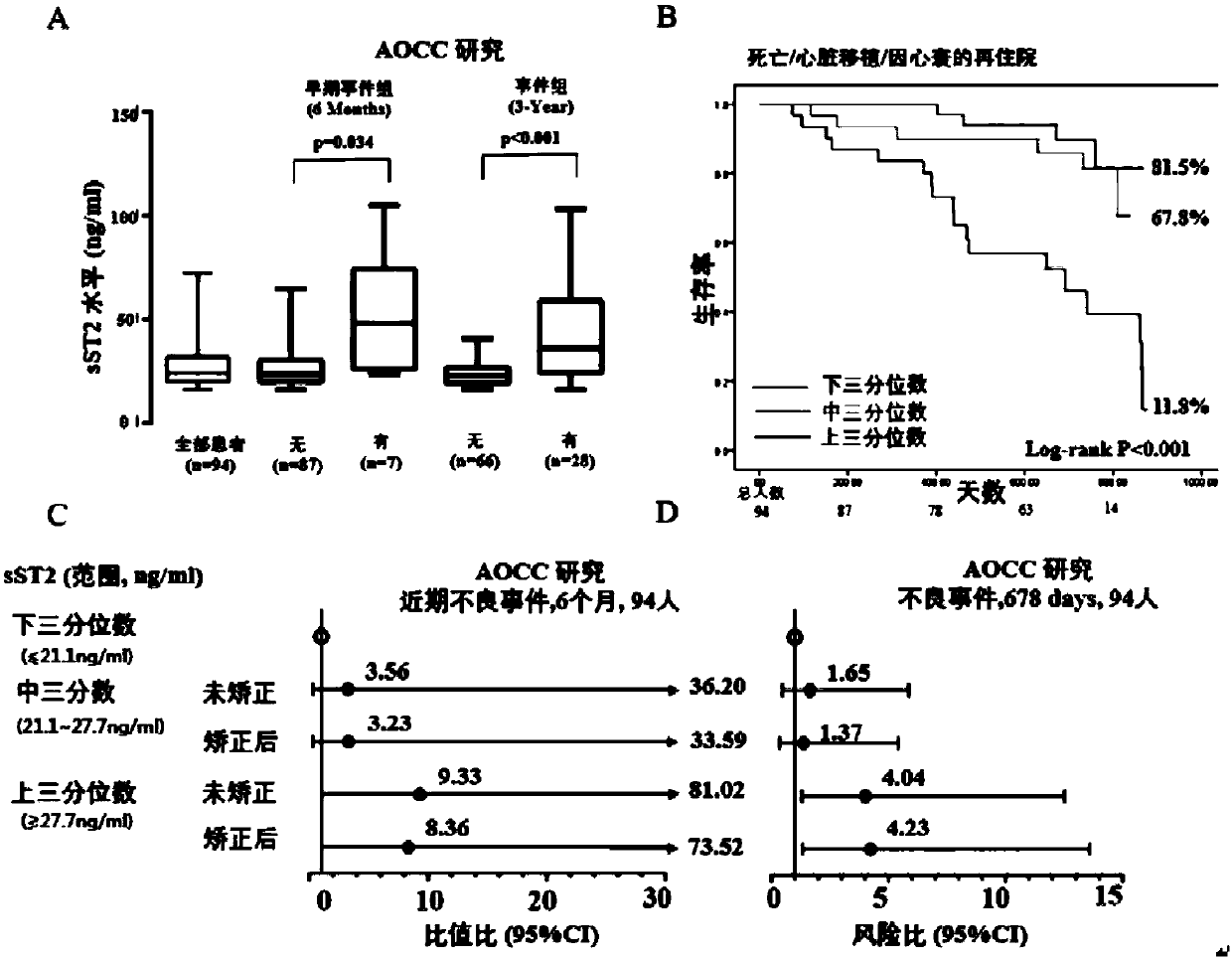
[0065] Example 1: Correlation between the level of serum sST2 and adverse events in children with dilated cardiomyopathy (DCM)
[0066] 1. Patient enrollment conditions and follow-up strategy
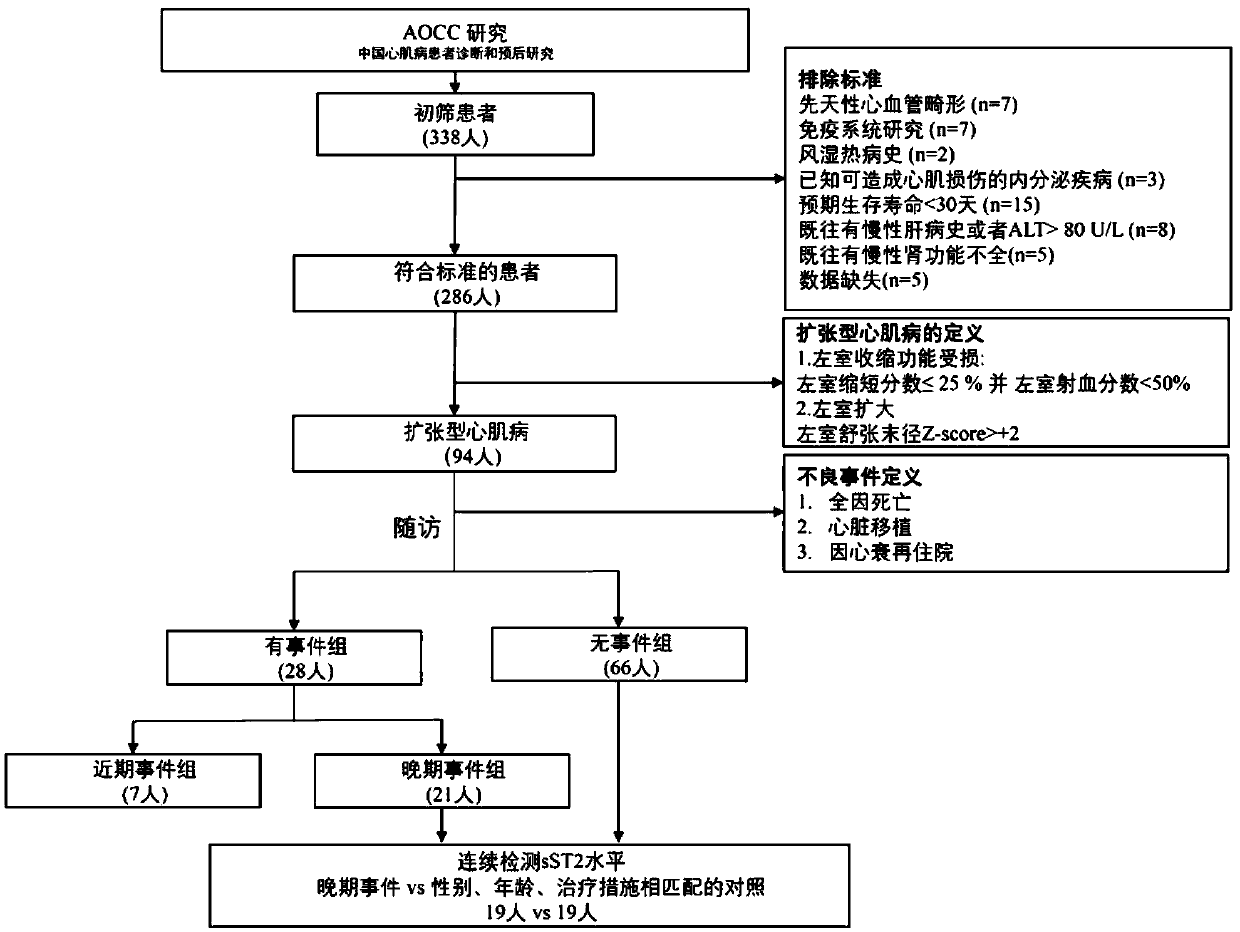
[0067] figure 1 A flow diagram of the study of the present invention is shown. The AOCC Study (Chinese Cardiomyopathy Diagnosis and Prognosis Study) is a double-center, observational, prospective, comprehensive, multi-omics study. All children (<18 years old) were treated at Beijing Anzhen Hospital and Fuwai Hospital from September 2015 to March 2017.
[0068] Dilated cardiomyopathy and heart failure were diagnosed and identified by at least 3 experienced cardiologists. Dilated cardiomyopathy is defined as the presence of at least 2 of the following criteria: (1) symptomatic heart failure; (2) left ventricular or biventricular systolic dysfunction; (3) ventricular dysfunction not explained by abnormal loading or coronary artery disease expansion. Systolic dysfunction was defined a...
Embodiment 2
[0095] Example 2: Serum sST2 levels can improve the predictive ability of serum BNP to predict the occurrence of adverse events in children with DCM
[0096] It has been reported in the literature that BNP is an independent predictor of adverse events in children with DCM (Suthar D, etal. Pediatr Cardiol. 2018). The present invention further verifies whether sST2 can improve the predictive ability of BNP.
[0097] For the risk prediction of recent adverse events, adding serum sST2 levels to the analysis of BNP to predict the risk of adverse events in children with DCM resulted in a C-statistic from 0.697 (95% CI, 0.541-0.852; P< 0.05) increased to 0.812 (95% CI, 0.697-0.939; P<0.05); NRI was 0.204 (95% CI, 0.048-0.375), while IDI also showed a corresponding improvement, see Table 4 for details.
[0098] For the risk prediction of long-term adverse events, increasing the serum sST2 level to BNP to predict the risk of adverse events in children with DCM, the C-statistic (C-stat...
Embodiment 3
[0106] Example 3: Continuous monitoring of serum sST2 levels can be used to monitor the course of disease in children with DCM
[0107] Previous studies have found that the increase in the level of sST2 precedes the occurrence of adverse events, suggesting that sST2 may be used for prognosis and detection of disease in clinical practice (van Vark LC et al. J Am Coll Cardiol. 2017). In order to avoid the confounding offset caused by children's development to the greatest extent, the present invention uses a nested case-control (Sadek AA, et al. Electron Physician. 2017) to evaluate the predictive value of repeated measurement of serum sST2.
[0108] In addition to the 7 children with recent adverse events, 21 children with DCM had adverse events (defined as late events) after 6 months of enrollment, including 19 children at 3 months and 6 months. Serum samples were collected during the follow-up visits; 19 children with consistent gender, age and anti-DCM treatment intervention...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com