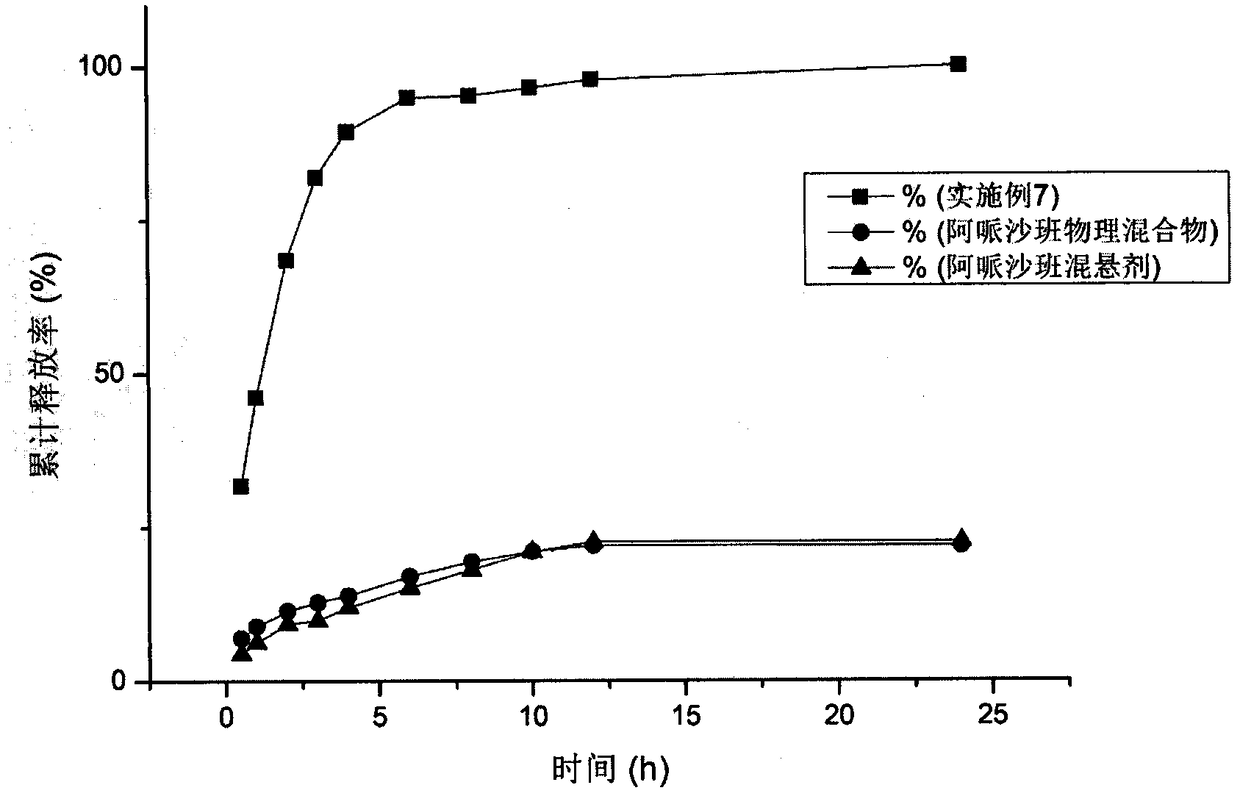
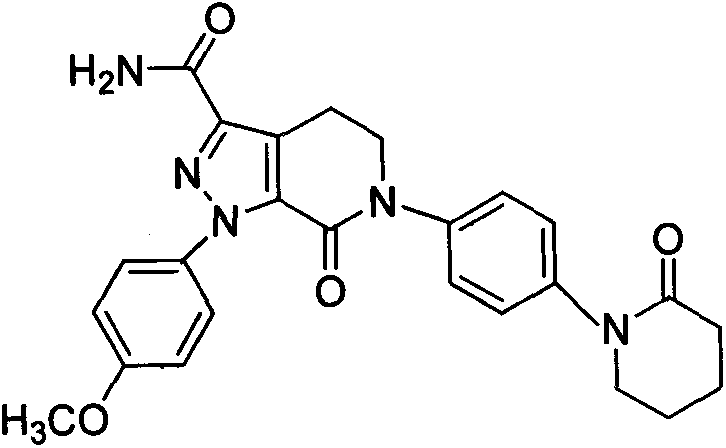
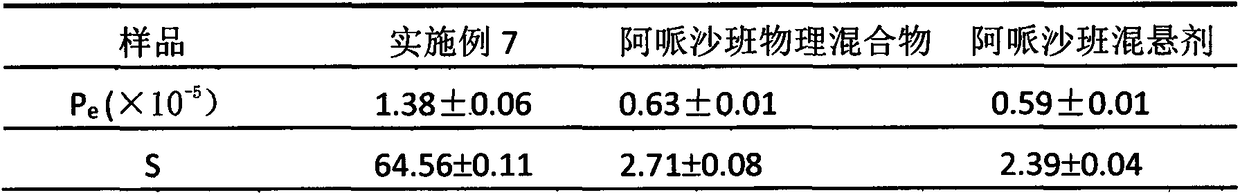
Apixaban nanometer mixed suspension and preparation method thereof
A nano-suspension, apixaban technology, applied in the field of medicine, can solve the problem of low bioavailability, achieve the effect of less dosage, increase bioavailability, and improve drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0026] Example 1
[0027] Weigh 20mg apixaban in 35ml water, ultrasonically treat it for 10min to fully disperse the drug in the water; shear it with a high-speed disperser at 10000rpm for 5min to obtain a crude suspension; homogenize the above-mentioned crude suspension under high pressure , First homogenize 2 times at 800bar, and then homogenize 12 times at 1800bar pressure to obtain apixaban nano suspension. The measured particle size of the nanosuspension is 754nm, and the polydispersity index (PDI) is 0.378.
Example Embodiment
[0028] Example 2
[0029] Weigh 5mg PVPK30 in 50ml water, add 20mg apixaban under stirring, and ultrasonically treat for 10min to fully disperse the drug in the water; shear with a high-speed disperser at 10000rpm for 5min to obtain a crude suspension; The suspension was homogenized at high pressure, firstly at 800 bar for 2 times, and then at 1800 bar for 12 times to obtain apixaban nano suspension. The measured particle size of the nanosuspension was 386.7 nm, and the polydispersity index (PDI) was 0.167.
Example Embodiment
[0030] Example 3
[0031] Weigh 1mg sodium taurocholate into 35ml water, add 20mg apixaban under stirring, and ultrasonically treat for 10min to fully disperse the drug in the water; shear with a high-speed disperser at 10000rpm for 5min to obtain a crude suspension; The above crude suspension was homogenized under high pressure, firstly at 800 bar for 2 times, and then at 1800 bar for 12 times to obtain apixaban nano-suspension. The particle size of the nanosuspension was measured to be 401.6 nm, and the polydispersity index (PDI) was 0.11.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap