A kind of microneedle patch and preparation method thereof
A micro-needle sticker and micro-needle technology, applied in the directions of micro-needle, needle, sheet delivery, etc., can solve the problems of residual harmful substances, poor drug delivery effect, and small drug load of coated micro-needles, and achieve good skin repair. and beauty, wrinkle reduction, toughness enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
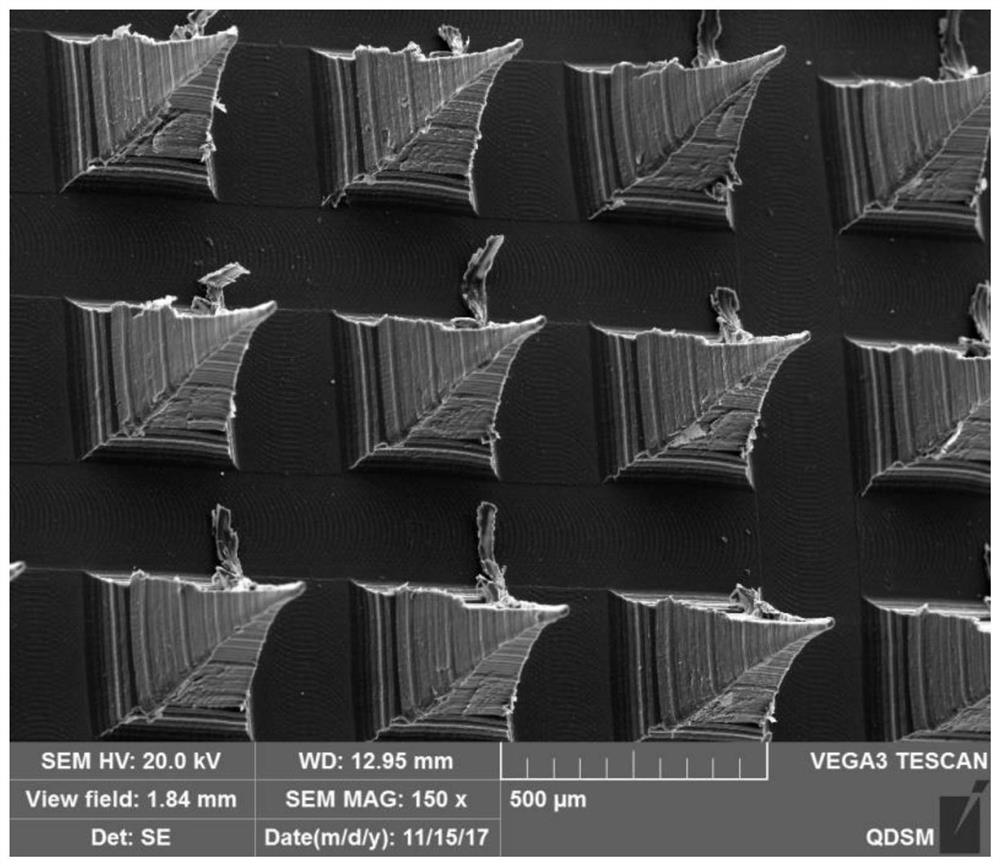
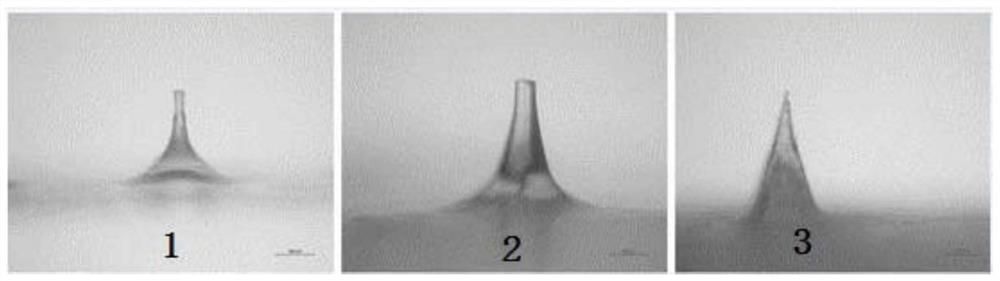
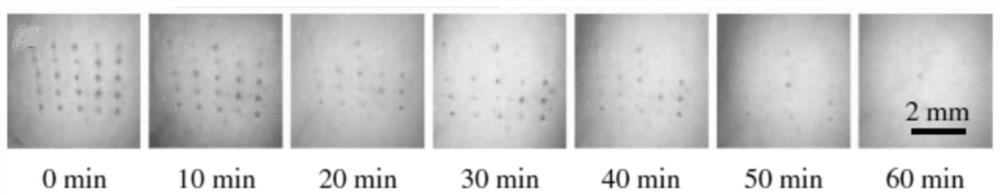
[0036] The present invention also provides a preparation method for preparing the above-mentioned microneedle patch, which includes: injecting the polymer solution into the micropore cavity of the microneedle mould, by vacuum jetting, and obtaining the needle layer after the polymer solution is solidified. The first side is coated with a dissolvable adhesive, and then a layer of gelatin is poured in. After the gelatin is formed, the cured microneedle patch is taken out from the microneedle mold.
[0037] Specifically, a preparation method of a microneedle patch mainly includes the following steps:
[0038] 1. Preparation of polymer solution
[0039] Dissolve the degradable polymer material in deionized water, prepare an aqueous solution with a mass concentration of 30-50 wt% degradable polymer material, and then add cosmetic functional molecules and reinforcements to the aqueous solution of the degradable polymer material to obtain a polymer solution, the prepared polymer solut...
Embodiment 1
[0048] The embodiment of the present invention provides a microneedle patch and a preparation method thereof.
[0049] 1. Preparation of polymer solution
[0050] Dissolving water-soluble polylactic acid with a molecular weight of 100,000 in deionized water to prepare an aqueous solution with a mass concentration of 40 wt% degradable polymer materials;
[0051] According to parts by weight, an aqueous solution of 5 parts of hyaluronic acid, 0.7 parts of carboxymethyl cellulose, 0.7 parts of collagen, 0.7 parts of EGF epidermal growth factor, 1.5 parts of calcium sulfate nano whiskers and 100 parts of degradable polymer materials The polymer solution is obtained by mixing, and the obtained polymer solution is subjected to centrifugation and vacuum filtration for subsequent use.
[0052] 2. Prepare needle layer
[0053] The polymer solution was injected into the microporous cavity of the microneedle mold by vacuum injection, and the microneedle mold injected with the polymer s...
Embodiment 2
[0057] The embodiment of the present invention provides a microneedle patch and a preparation method thereof.
[0058] 1. Preparation of polymer solution
[0059] Dissolving water-soluble polylactic acid with a molecular weight of 200,000 in deionized water to prepare an aqueous solution with a mass concentration of 40 wt% degradable polymer materials;
[0060] According to parts by weight, 5 parts of hyaluronic acid, 0.7 part of carboxymethyl cellulose, 0.7 part of collagen, 0.7 part of EGF epidermal growth factor, 0.7 part of kojic acid, 0.7 part of hydroquinone, 0.7 part of arbutin, 0.7 part of AHA, 0.7 vitamin C, 0.7 vitamin E, 0.7 N-acetylglucosamine, 0.7 tranexamic acid, 0.7 L-cysteine, 0.7 glutathione, 0.7 all-trans Retinoic acid, 0.7 part of adenosine, 0.7 part of endothelin antagonist, 0.7 part of mulberry tree extract, 0.7 part of licorice extract, 0.7 part of aloe extract, 1.5 part of calcium sulfate nano whiskers and 100 parts of degradable polymer materials The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com