Cyclic polypeptide radiopharmaceutical for integrin alpha v beta 6 targeting and preparation method thereof
A technology of radiopharmaceuticals and cyclic polypeptides, which is applied in the direction of radioactive carriers, can solve problems such as instability and poor tumor imaging effects, and achieve the effects of reducing the number of amino acids, increasing in vivo stability, and reducing synthesis costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
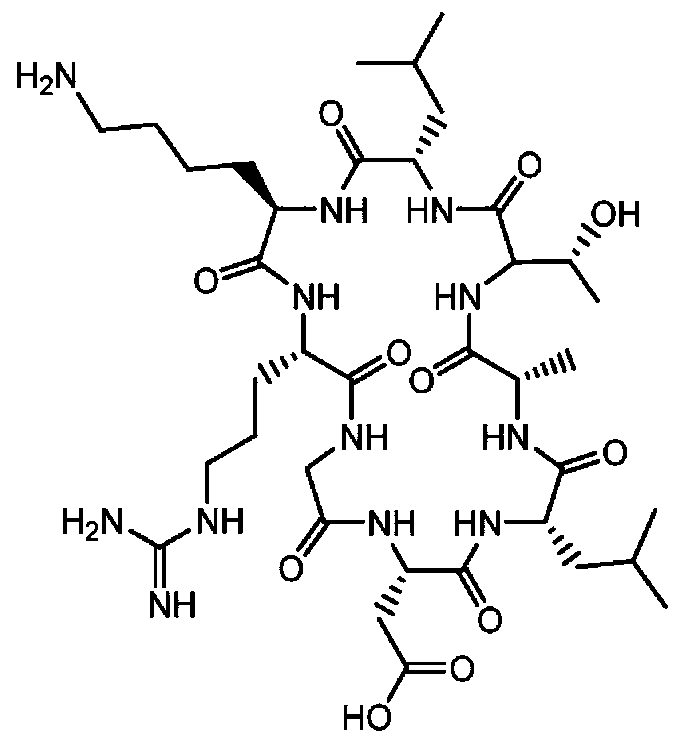
[0030] The cyclic polypeptide radiopharmaceutical for integrin αvβ6 targeting is a radionuclide-bifunctional chelating agent-cyclatide polypeptide, and the radionuclide is labeled with a bifunctional chelating agent for the cycratide polypeptide. The structure of the cycratide polypeptide is shown in formula (1):
[0031]
[0032] The preparation method of this compound:
[0033] Include the following steps:
[0034] a. Preparation of bifunctional chelating agent-cycratide polypeptide: dissolve cycratide polypeptide in alkaline buffer, add bifunctional chelating agent, mix and react at room temperature for 3-10 hours, separate and purify the reaction mixture by chromatography, and collect product peaks The product, freeze-dried to obtain a white powder that is a bifunctional chelating agent-cycratide polypeptide;
[0035] b. Preparation of radionuclide-bifunctional chelating agent-cycratide polypeptide: add radionuclide to the weakly acidic buffer of the bifunctional chelati...
Embodiment 2
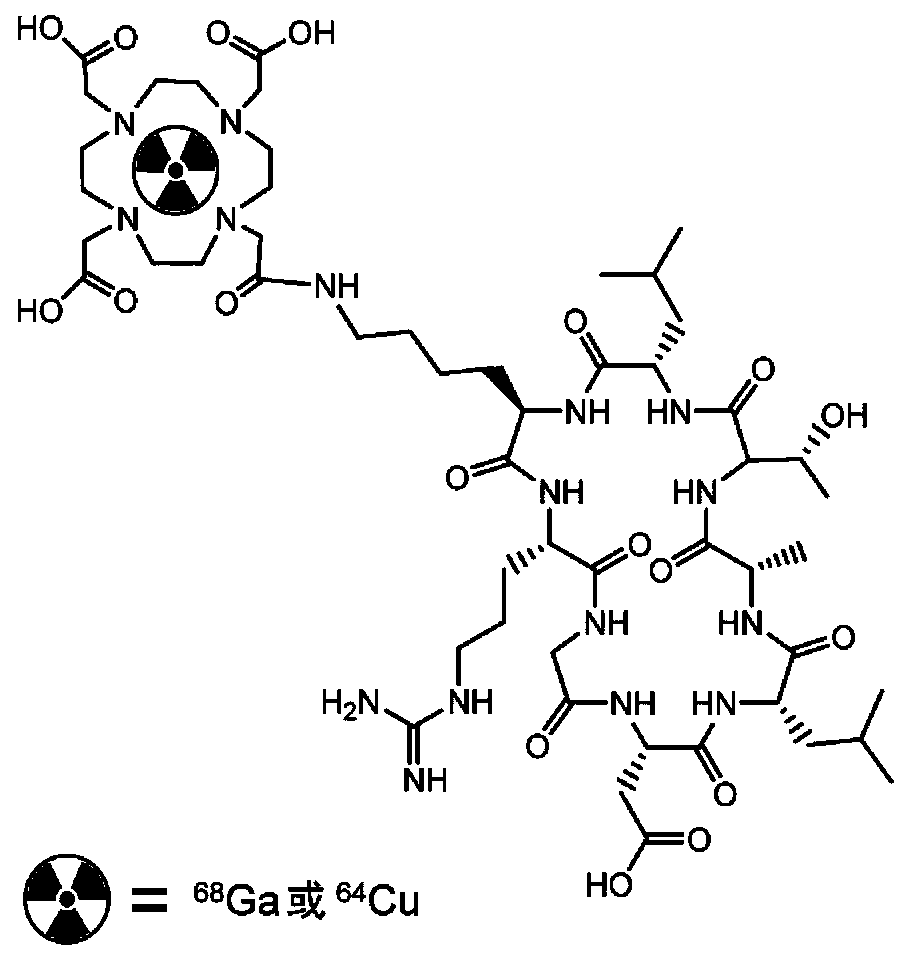
[0046] A cyclic polypeptide radiopharmaceutical for integrin αvβ6 targeting, wherein: the bifunctional chelator is DOTA-NHS or p-SCN-Bn-NOTA, and the radionuclide is 68 Ga or 64 The preparation method of Cu, cyclatide polypeptide is as in Example 1.
[0047] Preparation method of cyclic polypeptide radiopharmaceutical for integrin αvβ6 targeting:
[0048] In step a, the bifunctional chelating agent is DOTA-NHS or p-SCN-Bn-NOTA, and the specific operation steps are: take the cycratide polypeptide and dissolve it in 500 μL of 0.1mol / L NaHCO 3 buffer (pH=8.5-9.0), and then add bifunctional chelating agent (dissolved in DMF). After mixing, react at room temperature for 5 h, and then inject the reaction mixture into semi-preparative HPLC for separation and purification. The product peaks were collected, and acetonitrile was removed by rotary evaporation, and then freeze-dried into a white powder by a lyophilizer;
[0049] In step b, the radionuclide is 68 Ga or 64 Cu, the spe...
Embodiment 3
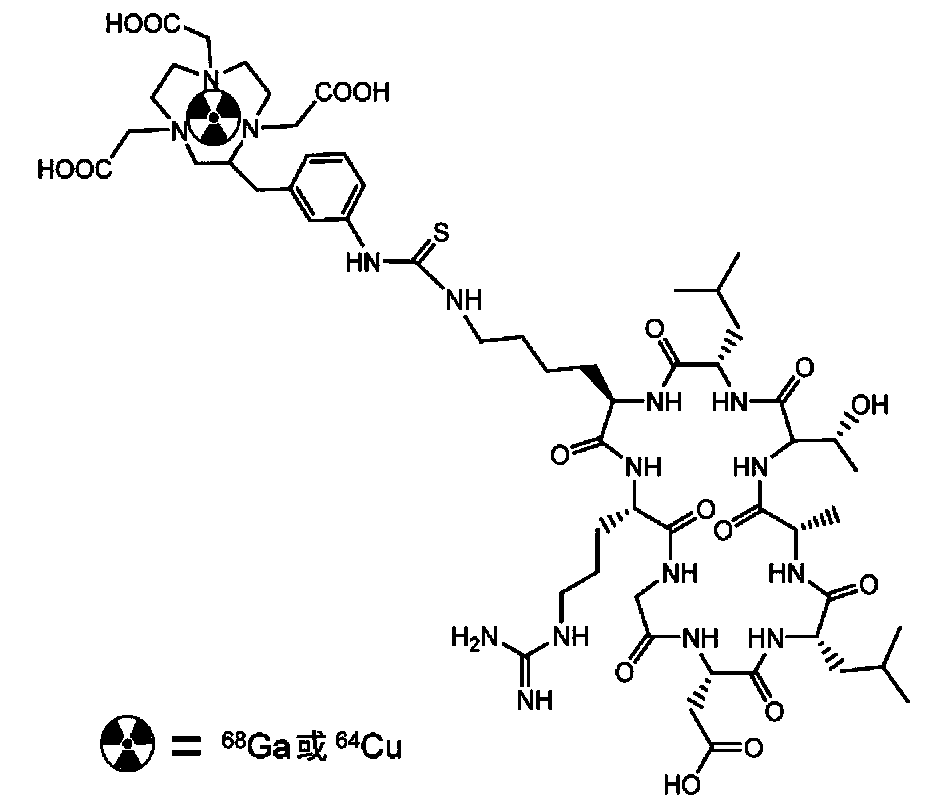
[0054] Cyclic polypeptide radiopharmaceuticals for integrin αvβ6 targeting, wherein: the bifunctional chelator is DOTA or NOTA or derivatives thereof, and the radionuclide is 68 Ga or 64 The preparation method of Cu, cyclatide polypeptide is as in Example 1.
[0055] Preparation method of cyclic polypeptide radiopharmaceutical for integrin αvβ6 targeting:
[0056] In step a, the bifunctional chelating agent is DOTA or NOTA or its derivatives, and the specific operation steps are: after mixing the bifunctional chelating agent with EDC and SNHS in a certain molar ratio, react at 4°C for 0.5-2h to activate. Take the cycratide polypeptide and dissolve it in 500 μL of 0.1mol / L NaHCO 3 buffer solution (pH=8.5-9.0), and then add the activated bifunctional chelating agent. After mixing, react at room temperature for 5 h, and then inject the reaction mixture into semi-preparative HPLC for separation and purification. The product peaks were collected, and acetonitrile was removed by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com