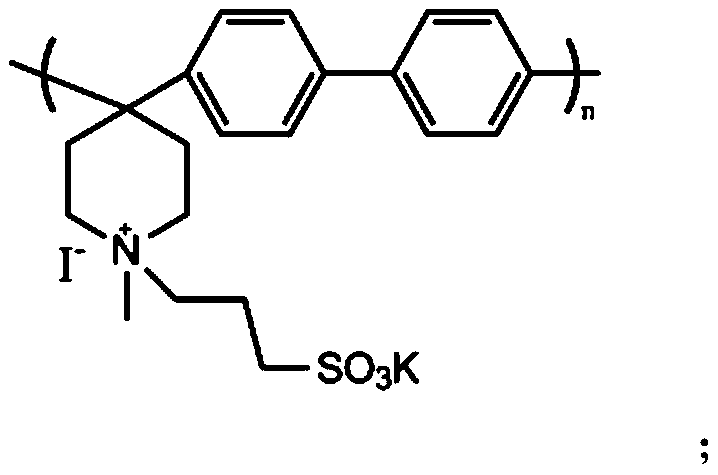
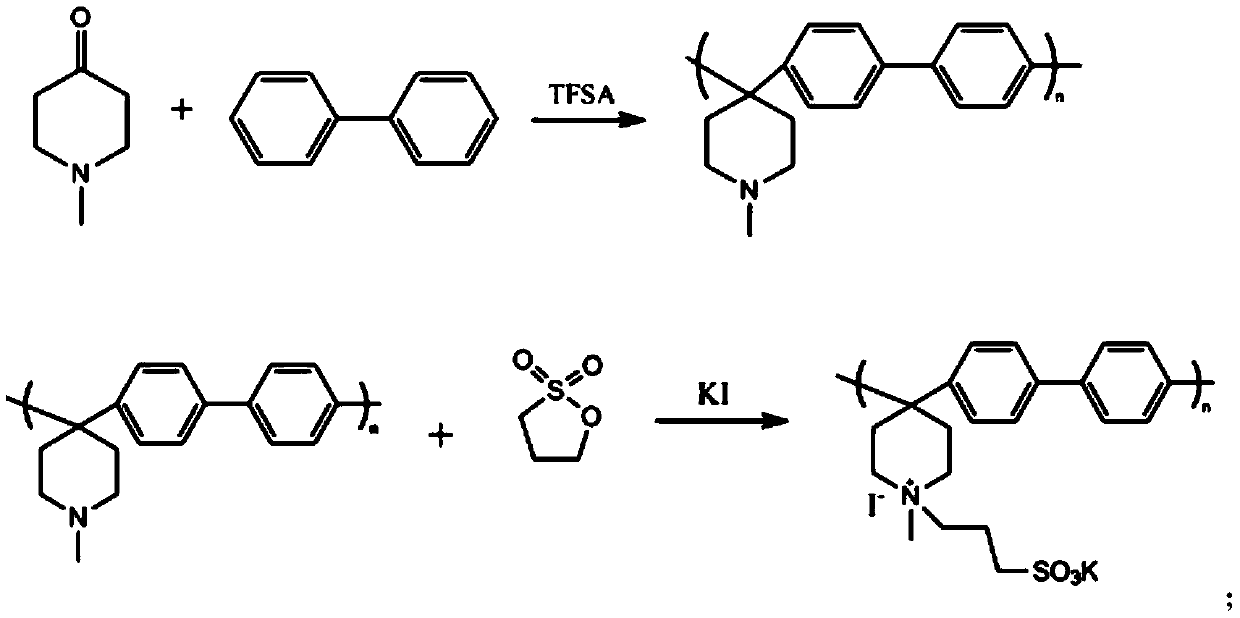
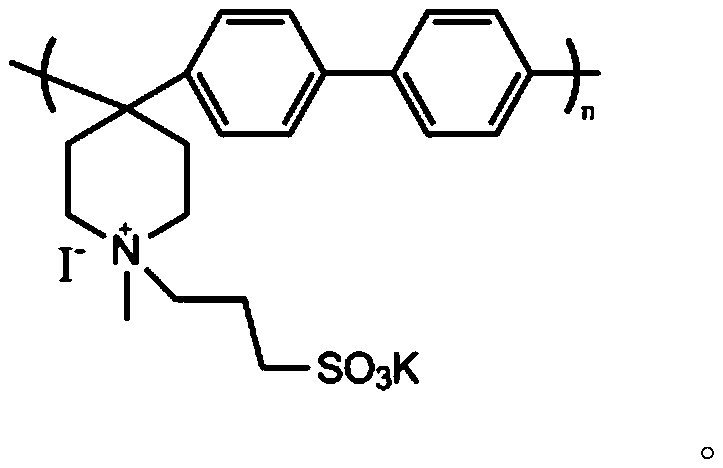
Polyarylene piperidine amphoteric ion exchange membrane without aryl ether bond and preparation method thereof
A polyarylpiperidine and zwitterion technology, which is applied to polyarylpiperidine-based zwitterion exchange membranes without aryl ether bonds and the field of preparation thereof, can solve the problem of unstable aryl ether bond structure and cracking of polymer main chains. and other problems to achieve the effect of improving vanadium resistance, good stability and high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 2.5 g of biphenyl was dissolved in 6 mL of dichloromethane, then 2.23 mL of N-methyl-4-piperidone was added. In order to avoid violent fuming after the addition of strong acid, 1.21 mL of trifluoroacetic acid was added dropwise under conditions of ice-water bath and nitrogen gas, followed by 14.4 mL of trifluoromethanesulfonic acid. With the addition of trifluoromethanesulfonic acid, the solution turned from yellow via orange / red to a brown homogeneous solution, which remained so during the reaction. During the polymerization reaction, the reaction vessel was placed in an ice bath to suppress the occurrence of side reactions. After 3 hours of reaction, when the viscosity of the reactant increased to the point where mechanical stirring was difficult, the reaction was stopped. First, in the reaction vessel, slowly add dimethyl sulfoxide to dissolve the polymerized reaction product main chain polymer, then take 5M NaOH solution (500-1000mL) in a beaker, and slowly pour th...
Embodiment 2
[0026] The main chain polymer was prepared as described in Example 1. 1.0 g of the main chain polymer was added to 30 mL of dimethyl sulfoxide and stirred to form a white emulsion. Feed propane sultone, KI and the main chain polymer at a ratio of 0.1:0.1:1, stir at 60°C for 48 hours, and react to obtain a uniform orange solution. The solution was slowly poured into the stirred ethyl acetate solution to precipitate a zwitterionic polymer with a graft degree of 10%.
[0027] The product was washed several times with ethyl acetate, filtered with suction, and dried in a constant temperature drying oven. Dissolve 0.15 g of amphoteric polymer in 4 mL of dimethyl sulfoxide, and cast at 50° C. for 24 h to form a film. The membrane was soaked in deionized water for 12 h at room temperature to remove impurities. Then, the membrane was soaked in acid for 12h to make it fully ion exchanged. The membrane is then soaked in deionized water to remove excess acid. The water absorption of t...
Embodiment 3
[0029] The main chain polymer was prepared as described in Example 1. 1.0 g of the main chain polymer was added to 30 mL of dimethyl sulfoxide and stirred to form a white emulsion. Feed propane sultone, KI and the main chain polymer at a ratio of 0.2:0.2:1, stir at 60°C for 48 hours, and react to obtain a uniform orange solution. The solution was slowly poured into the stirred ethyl acetate solution to precipitate a zwitterionic polymer with a graft degree of 20%.
[0030]The product was washed several times with ethyl acetate, filtered with suction, and dried in a constant temperature drying oven. Dissolve 0.15 g of amphoteric polymer in 4 mL of dimethyl sulfoxide, and cast at 50° C. for 24 h to form a film. The membrane was soaked in deionized water for 12 h at room temperature to remove impurities. Then, the membrane was soaked in acid for 12h to make it fully ion exchanged. The membrane is then soaked in deionized water to remove excess acid. The water absorption of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com