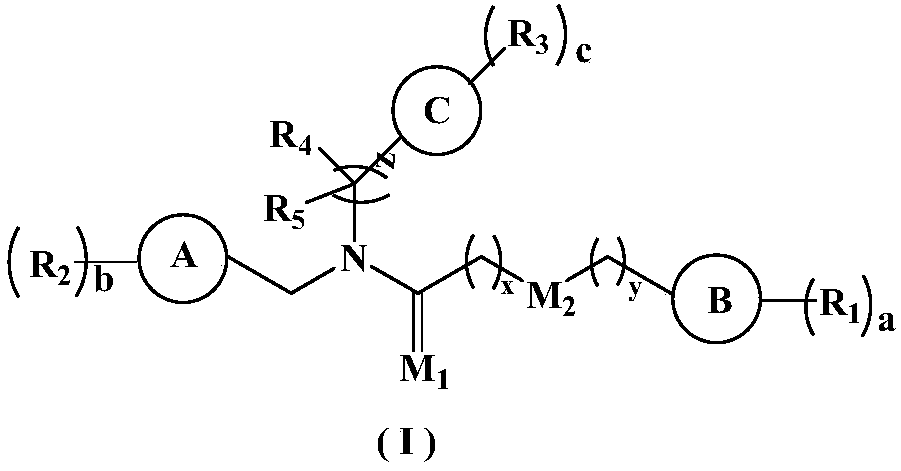
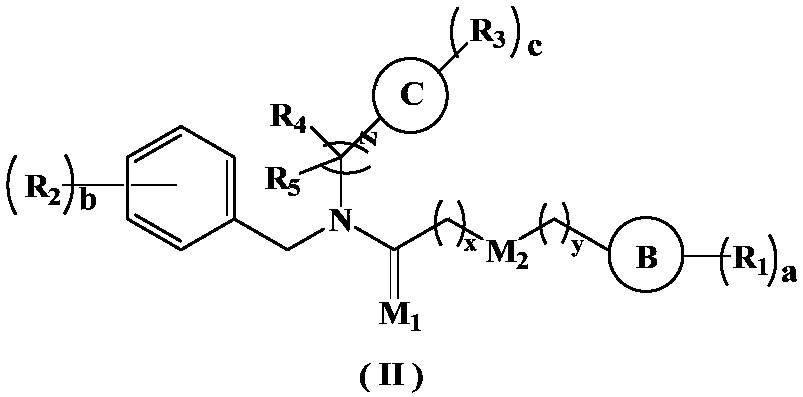
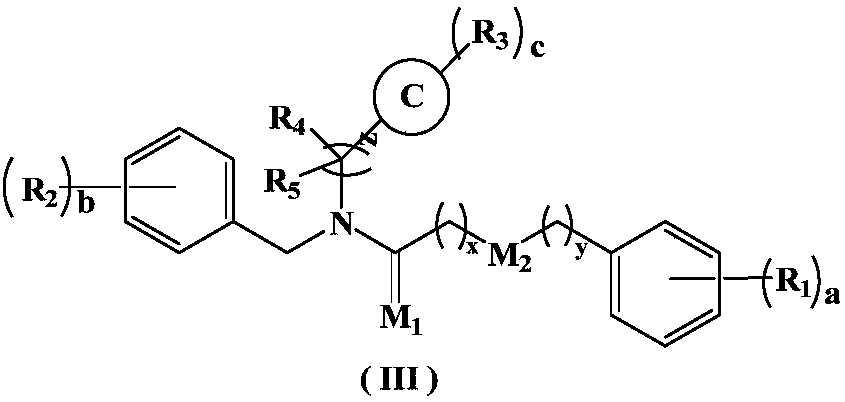
5-HT2A receptor inhibitor, preparation method and applications thereof
A technology that accepts salts and stereoisomers, applied in the field of drug development, can solve the problems of unavoidable side effects of extrapyramidal tract and weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-((1-isopropylazetidin-3-yl)methyl)urea
[0146]
[0147] The first step: the synthesis of benzyl (1-isopropyl azetidin-3-yl) methyl carbamate
[0148]
[0149] At room temperature, dissolve benzyl (azetidin-3-ylmethyl) carbamate (0.4g, 1.82mmol) and acetone (0.16g, 2.72mmol) in DCE (10mL), stir for one hour, then add in portions Sodium triacetylborohydride (0.58g, 102.9mmol), stirred at room temperature for 18 hours, then washed with CH 2 Cl 2 (50mL), diluted with diatomaceous earth, the organic phase was washed with water (10mL) and saturated brine (15mL) successively, and dried with anhydrous sodium sulfate, column chromatography after concentration gave compound benzyl (1-isopropylacridine Butidin-3-yl)methylcarbamate (0.21 g, 44%).
[0150] MS m / z(ESI):263.2[M+H] + .
[0151] The second step: the synthesis of (1-isopropylazetidin-3-yl)methanamine
[0152]
[0153] To a solution of benzyl(1-isopropylazetidin-3...
Embodiment 2
[0165] 1-((1-ethylazetidin-3-yl)methyl)-1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)urea
[0166]
[0167] The preparation method of 1-((1-ethylazetidin-3-yl)methyl)-1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)urea refers to the implementation example 1.
[0168] 1 H NMR (400MHz, CDCl 3 )δ7.24-7.08(m,4H),7.03-6.94(m,2H),6.84-6.74(m,2H),4.43(s,2H),4.34(d,J=4.34Hz,2H),3.84 (m,2H),3.69(m,6H),3.04-2.84(m,3H),2.13-1.96(m,1H),1.24-1.15(t,J=14.5Hz,3H),1.01(d,J =6.7Hz,6H);
[0169] MS m / z(ESI):428.2[M+H] + .
Embodiment 3
[0171] 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-((1-methylazetidin-3-yl)methyl)urea
[0172]
[0173] The preparation method of 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-((1-methylazetidin-3-yl)methyl)urea refers to the implementation example 1.
[0174] 1 H NMR (400MHz, CDCl 3 )δ7.18-7.13(m,4H),7.03-6.96(m,2H),6.85-6.79(m,2H),5.30-5.19(m,1H),4.44(s,2H),4.34(d, J=5.4Hz, 2H), 3.69(d, J=6.6Hz, 2H), 3.50(d, J=7.7Hz, 2H), 3.29-3.24(m, 2H), 3.02-2.96(m, 2H), 2.65-2.57(m,1H),2.28(s,3H),2.09-2.03(m,1H),1.01(d,J=6.7Hz,6H);
[0175] MS m / z(ESI):414.3[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com