A method for extracting valuable metals from lithium-ion battery mixed manganese-rich waste leaching solution
A technology for lithium-ion batteries and valuable metals, applied in battery recycling, manganese sulfate, recycling by waste collectors, etc., can solve the problems of lithium-ion battery recycling, long nickel and cobalt separation process, and large manganese extraction load. Achieve the effects of low cost, short process and small amount of sediment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
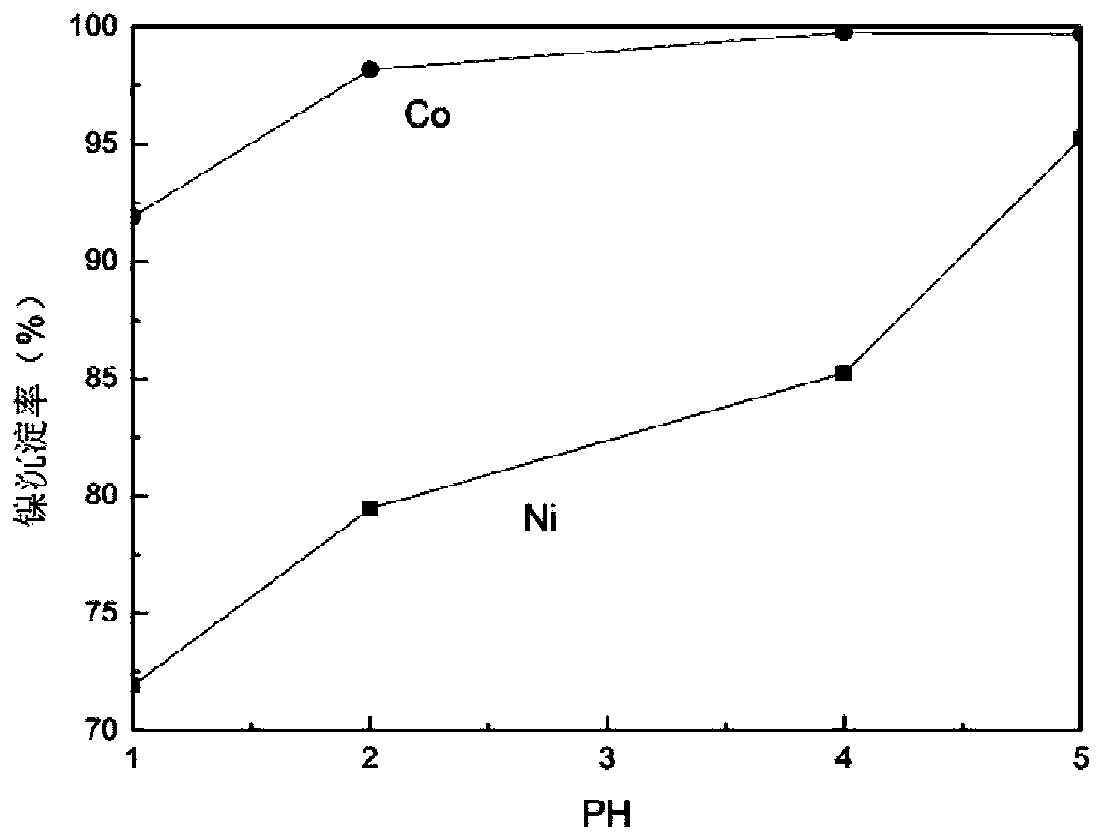
[0035] Take 100mlLiMn 2 o 3 with LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2The sulfuric acid leaching solution of mixed material is placed in the round bottom flask of 250ml, and the content of nickel, cobalt, manganese, lithium in the solution is as shown in table 1. Heat the round bottom flask in a water bath at 50°C, add sodium xanthate to the solution, control the molar ratio of sodium ethyl xanthate to nickel-cobalt in the solution to 2.3:1, and use a magnetic force of 150 rpm Under stirring condition, react for 2 hours. In this reaction process, the pH value is an extremely critical factor, and the relationship between the nickel-cobalt precipitation rate and the pH value is as follows: figure 1 shown. As the pH value continues to increase, the precipitation rate of nickel and cobalt continues to increase, but when the pH value exceeds 5, the loss rate of manganese in the solution becomes significantly higher. In order to effectively control the content of manganese in the precip...
Embodiment 2
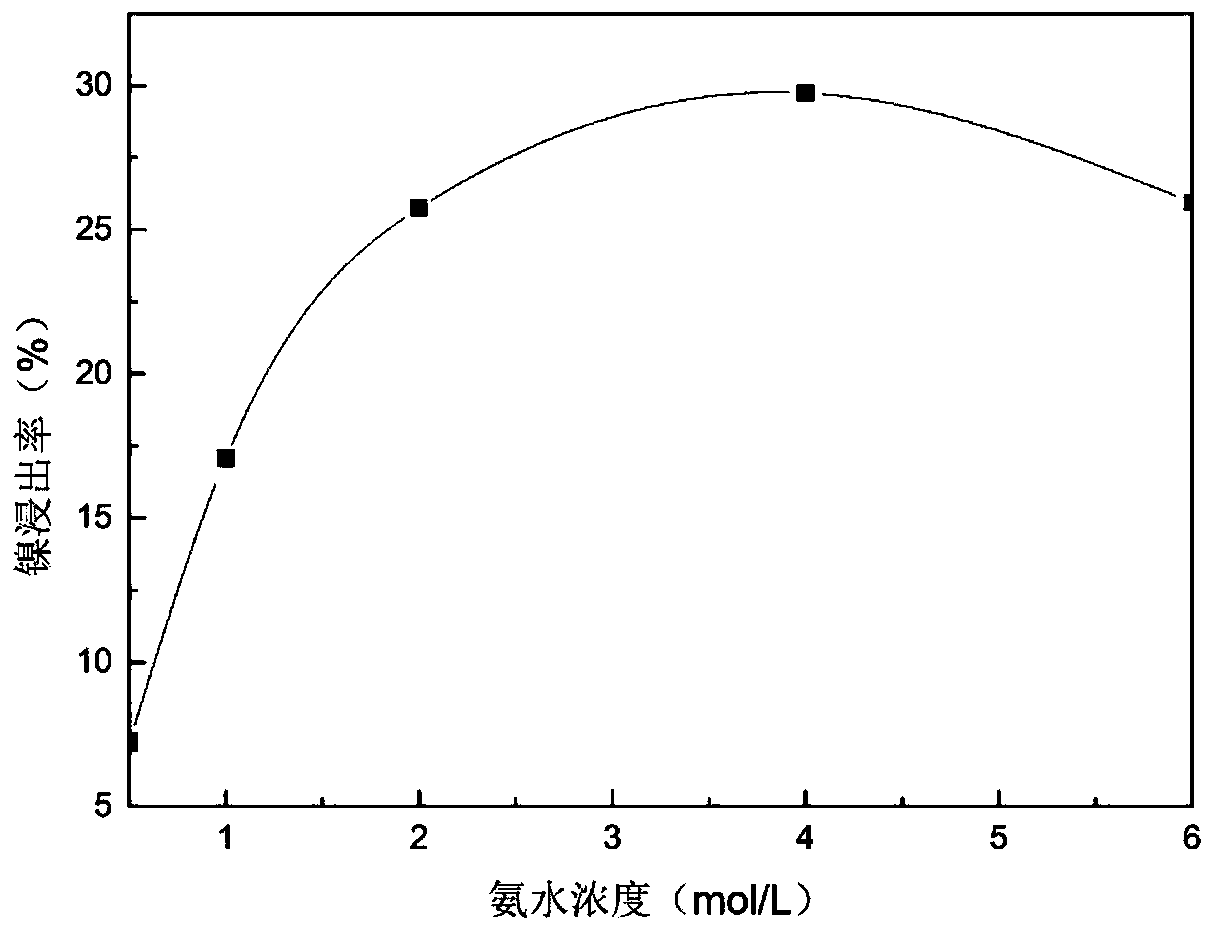
[0041] The leaching solution used in this example is the same as in Example 1. The precipitant for separating nickel and cobalt is ethyl xanthic acid. The reaction steps and conditions are the same as in Example 1. The results are similar and will not be repeated. Take 10 g of the precipitate and place it in 60ml of 4mol / L ammonia water and 0.5mol / L ammonium sulfate mixed solution, under the condition of room temperature 25 ℃, react for 1 hour. The leaching rate of nickel is above 98%, while cobalt does not dissolve.
[0042] Take 20ml of sodium xanthate precipitation to remove nickel and cobalt and then filter the filtrate to place in a 150ml separating funnel. Use mono-2-ethylhexyl ethylhexylphosphate as the extractant, dissolve it in sulfonated kerosene at a volume ratio of 1:1, then measure 20ml of the dissolved extractant solution, and add it to an equal volume of filtrate. Under the condition of 25°C, shake and extract on a shaker for 10 minutes. The relationship betwe...
Embodiment 3
[0046] The leaching solution used in this example is the same as Example 1. The precipitating agent that separates nickel and cobalt is sodium formazate, and its reaction steps and reaction conditions are basically similar to embodiment 1 and 2, and difference is that the addition of precipitating agent sodium formamic and the relation of nickel-cobalt content are: the consumption of sodium formamic and nickel The molar ratio of cobalt is 3.5:1. The pH value is controlled at 5.0, and the results show that the precipitation rate of nickel and cobalt can reach more than 99%.
[0047] The process of the separation of manganese and lithium and the separation of nickel and cobalt in this embodiment are the same as the steps and conditions of embodiment 1. The effects of separation of nickel and cobalt and separation of manganese and lithium are also basically the same as in embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com