Thrombus-targeted long-cycle sustained release liposome and preparation method thereof
A long-circulation, slow-release lipid technology, applied in liposome delivery, biochemical equipment and methods, blood diseases, etc., can solve the problem of slow-release effect, unsatisfactory encapsulation rate and drug loading rate, and unsatisfactory thrombus targeting , drug release too fast and other problems, to achieve the effect of maintaining effective drug concentration, improving medication compliance, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Feeding: Prepare materials according to the quality of each material in Table 1
[0029] The feeding quality of each material in Table 1 and the percentage of the total mass (by preparing 100mg)
[0030]
[0031] 2. Preparation method
[0032]Step 1: Prepare drug-loaded long-circulation sustained-release liposome suspension by reverse-phase evaporation method: Weigh soybean phospholipids, cholesterol and star-shaped cholic acid functionalized polylactic acid, and dissolve them all in a certain amount of mixed solvent of chloroform and methanol (1:4, V / V); dissolve urokinase in barbiturate buffer (0.01mol / L, pH=7.4), add it to the above solution, mix and shake, and ultrasonicate in a water bath for 4 to 6 minutes to obtain The emulsion was rotated under reduced pressure on a rotary evaporator to remove the organic solvent, probe-type ultrasound for 1 min, added distearoylphosphatidylethanolamine-polyethylene glycol solution, and incubated in a water bath at 5-8°C ...
Embodiment 2
[0060] 1. Feeding: Prepare materials according to the quality of each material in Table 2
[0061] Table 2 The feeding quality of each material and the percentage of the total mass (based on the preparation of 100mg)
[0062]
[0063]
[0064] 2. Preparation method
[0065] The preparation method is the same as in Example 1.
[0066] 3. Characterization
[0067] Characterization observation method is the same as embodiment 1, and the results are as follows:
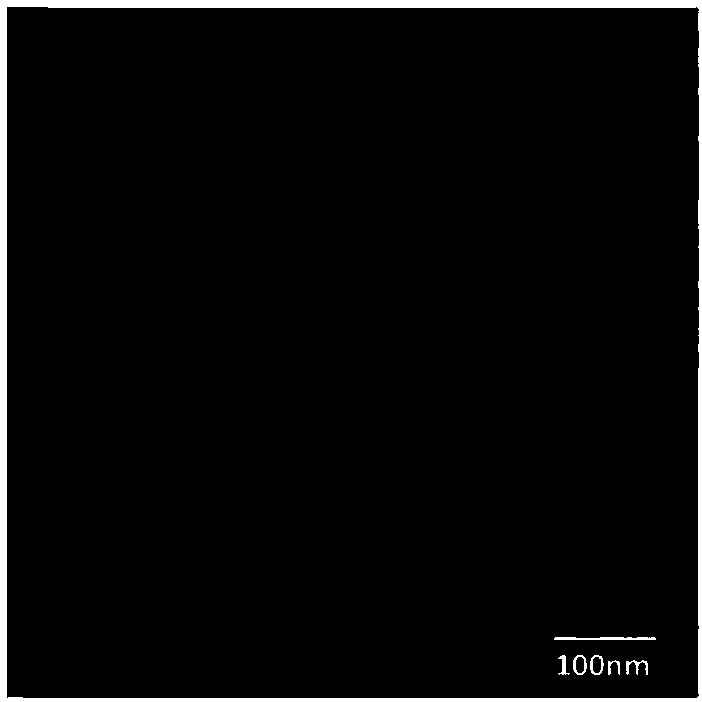
[0068] (1), the surface morphology is spherical, and the size distribution is relatively uniform. For details, see figure 2 .
[0069] (2), the average particle size is 91.5nm; the polydispersity coefficient is 0.203.
[0070] (3), encapsulation efficiency and drug loading
[0071] Determination results: the drug loading is 4.7%; the encapsulation efficiency is 80.6%. The drug-loading capacity of the liposome (other components are the same) without star-shaped cholic acid functionalized polylactic acid is 3....
Embodiment 3
[0081] 1. Feeding: Prepare materials according to the quality of each material in Table 3
[0082] Table 3 The feeding quality of each material and the percentage of the total mass (based on the preparation of 100mg)
[0083]
[0084] 2. Preparation method
[0085] Preparation method is the same as embodiment 1
[0086] 3. Characterization
[0087] Characterization observation method is the same as embodiment 1, and the results are as follows:
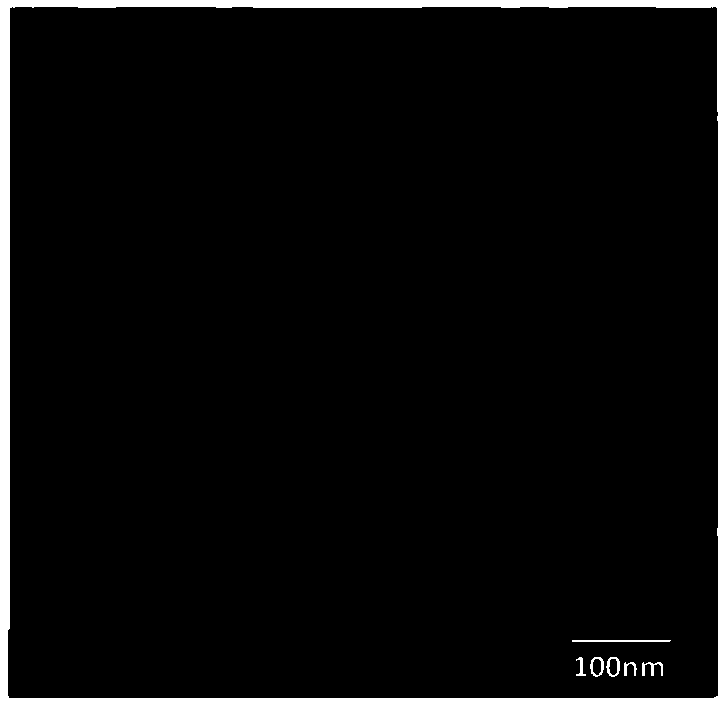
[0088] (1), the surface morphology is spherical, and the size distribution is relatively uniform. For details, see image 3 .
[0089] (2), the average particle size is 91.5nm; the polydispersity coefficient is 0.203.
[0090] (3), encapsulation efficiency and drug loading
[0091] Determination results: the drug loading is 6.3%; the encapsulation efficiency is 82.5%. The drug-loading capacity of the liposome without adding star-shaped cholic acid-functionalized polylactic acid (other components are the same) is 4.5%, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap