Method for potato leafroll virus (PLRV) detection, and special antibody adopted by method
A leaf-roll virus and potato technology, applied in the biological field, can solve the problems affecting potato quality and yield, compound infection, etc., and achieve the effects of great application value, good specificity and high accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]Embodiment 1, the preparation of polyclonal antibody
[0054] 1. Construction of recombinant plasmid pDB.His.MBP-PLRV-MP
[0055] 1. Using the RNA of PLRV as a template, the 5'- CATATG TCAATGGTGGTGTACAA-3' (the underline is the recognition site of restriction endonuclease Nde I) and 5'- CTCGAG TCCGCGCTTGATAAG-3' (the underline is the recognition site of the restriction endonuclease Xho I) was used as a primer for PCR amplification, and a PCR amplification product of about 480bp was obtained.
[0056] 2. Ligate the PCR amplification product obtained in step 1 with the cloning vector pMD19-T to obtain the recombinant plasmid pT-PLRV-MP.
[0057] 3. Take the recombinant plasmid pT-PLRV-MP, digest it with restriction endonucleases Nde I and Xho I, and recover a fragment of about 480 bp.
[0058] 4. Take the vector pDB.His.MBP, digest it with restriction endonucleases Nde I and Xho I, and recover the vector skeleton of about 6.5 kb.
[0059] 5. Ligate the restriction fr...
Embodiment 2
[0085] Embodiment 2, polyclonal antibody detects PLRV
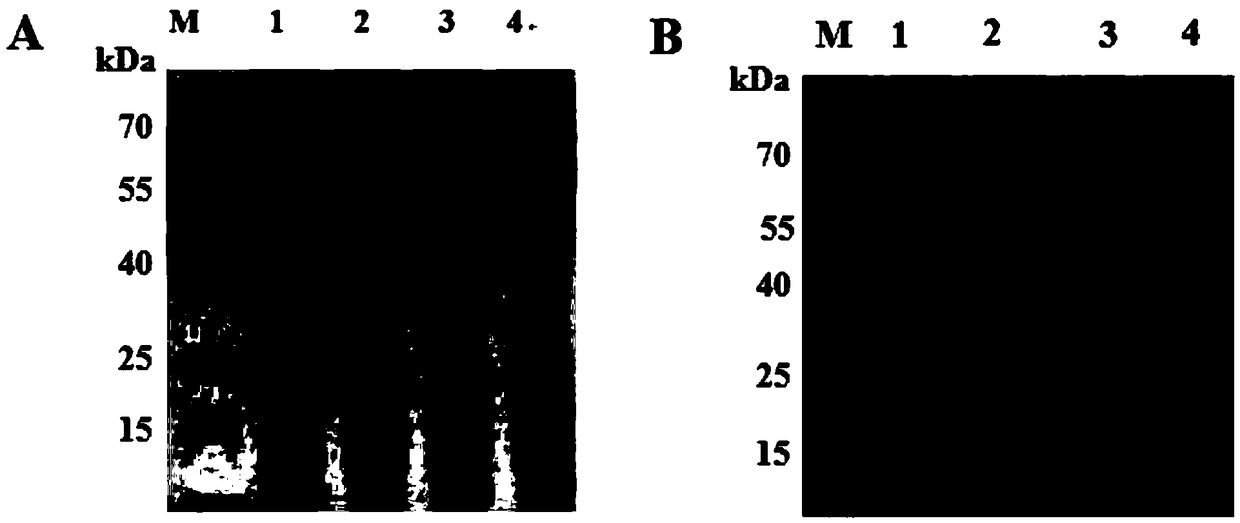
[0086] SDS-PAGE and Western blot were performed on the total protein of the leaves of the tobacco system infected with PLRV, and the polyclonal antibody prepared in step 3 of Example 1 was used as the primary antibody to detect PLRV. Specific steps are as follows:
[0087] 1. Extract the total protein of the leaves of the N. benthamiana system leaves infected by PLRV.
[0088] 2. After step 1 is completed, the total leaf protein is subjected to SDS-PAGE, and then the protein is transferred to a nitrocellulose membrane by electrotransfer (200mA, 85min).
[0089] 3. After completing step 2, place the nitrocellulose membrane in TBST buffer containing 5% (w / v) skimmed milk powder, and block at 37° C. for 1 hour.
[0090] 4. After completing step 3, add the dilution of the polyclonal antibody prepared in step 3 in Example 1, incubate at 37°C for 1 hour, and then wash the membrane with TBST buffer 3 times, 10 minutes each tim...
Embodiment 3
[0094] Embodiment 3, titer determination of polyclonal antibody
[0095] 1. Extract the total protein from leaves of N. benthamiana leaves infected with PLRV or healthy N. benthamiana leaves (as a negative control).
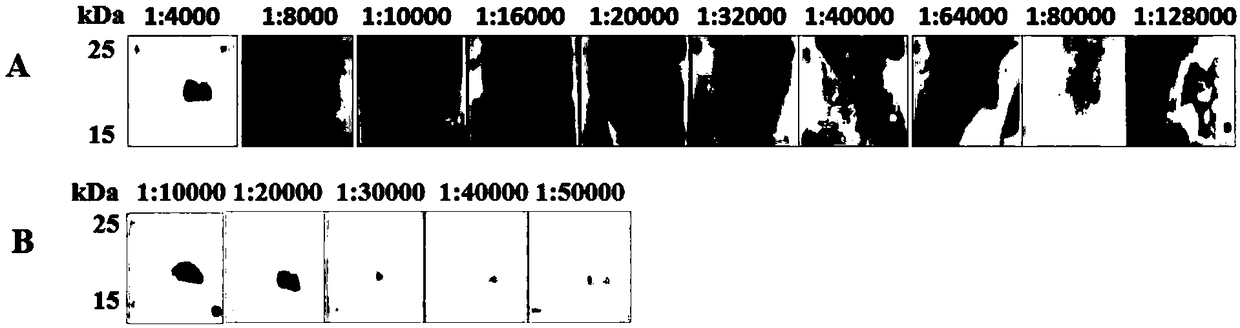
[0096] 2. Take the polyclonal antibody prepared in Step 3 of Example 1, and add TBST buffer for dilution to obtain a dilution. The dilution times of the diluent are 4000, 8000, 10000, 16000, 20000, 32000, 40000, 64000, 80000 and 128000, respectively.
[0097] 3. Perform SDS-PAGE and Western blot on the total protein extracted in step 1, and use the dilution obtained in step 2 as the primary antibody to detect PLRV.
[0098] For some Western blot results, see figure 2 In A (from left to right, the dilution ratios of the dilutions are 4000, 8000, 10000, 16000, 20000, 32000, 40000, 64000, 80000 and 128000).
[0099] 4. Extract the total protein from the inoculated leaves of N. benthamiana or healthy N. benthamiana leaves (as a negative control) infected by PLRV....
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap