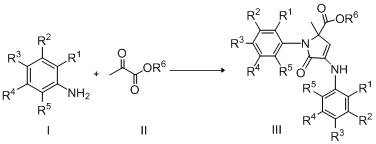
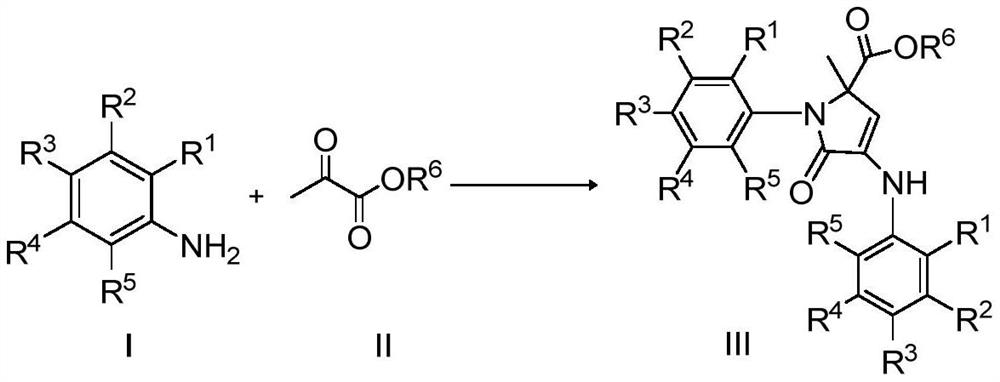
Catalytic synthesis of 3-pyrrolin-2-one in emulsion with titanocene dichloride and Bronsted acid
A technology of Bronsted acid and titanocene dichloride is applied in the field of synthesis of pyrrolinone compounds to achieve the effect of large reaction specific surface area, increase local concentration and avoid by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
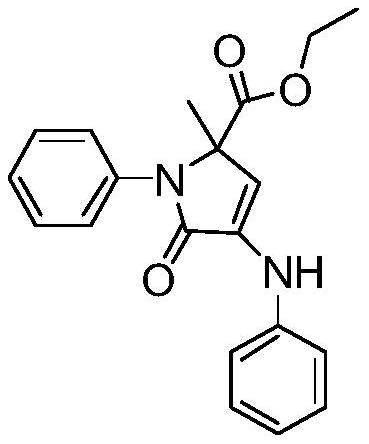
[0017] Synthesis of ethyl 2-methyl-5-oxo-1-phenyl-4-(phenylamino)-2,5-dihydro-1H-pyrrole-2-carboxylate with the following structural formula
[0018]
[0019] Add 181 μL (2 mmol) of aniline, 274 μL (2.5 mmol) of ethyl pyruvate, 0.01245 g (0.05 mmol) of titanocene dichloride, 0.01671 g (0.1 mmol) of p-nitrobenzoic acid, 1333 μL of distilled water, and 667 μL of chlorobenzene to the sample bottle, stirred and reacted at 50° C. for 6 hours, stopped the reaction, naturally cooled to room temperature, and separated by column chromatography to obtain solid 2-methyl-5-oxo-1-phenyl-4-(phenylamino)-2, 5-Dihydro-1H-pyrrole-2-carboxylic acid ethyl ester, its yield is 94%, and the structural characterization data are: 1 HNMR (600MHz, CDCl 3 )δ7.41(dt, J=8.7,7.8Hz,4H),7.37-7.31(m,2H),7.29(t,J=7.2Hz,1H),7.08(d,J=7.7Hz,2H), 7.00(t,J=7.4Hz,1H),6.68(s,1H),5.99(s,1H),4.20(dddd,J=25.0,10.8,7.1,3.7Hz,2H),1.66(s,3H) ,1.24(t,J=7.1Hz,3H); 13 C NMR (101MHz, CDCl 3 ( s), 117.03(s), 107.69(s),...
Embodiment 2
[0021] Synthesis of 1-(4-ethylphenyl)-4-((4-ethylphenyl)amino)-2-methyl-5-oxo-2,5-dihydro-1H-pyrrole- 2-Ethyl carboxylate
[0022]
[0023] In this example, the aniline in Example 1 was replaced with equimolar 4-ethylaniline, and the other steps were the same as in Example 1 to obtain solid 1-(4-ethylphenyl)-4-((4-ethylphenyl) Phenyl)amino)-2-methyl-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxylic acid ethyl ester, the yield is 93%, and the structural characterization data are: 1 H NMR (600MHz, CDCl 3 )δ7.20(d, J=8.4Hz, 2H), 7.16(d, J=8.4Hz, 2H), 7.07(d, J=8.3Hz, 2H), 6.93(d, J=8.4Hz, 2H) ,5.85(s,1H),4.16-4.03(m,2H),2.56(dq,J=31.7,7.6Hz,4H),1.55(s,3H),1.16(ddd,J=12.8,9.2,6.7Hz ,10H); 13 C NMR (151MHz, CDCl 3 )δ171.72(s),167.58(s),143.04(s),138.79(s),137.57(s),133.97(s),133.45(s),128.72(s),128.57(s),125.75( s), 117.11(s), 106.86(s), 68.74(s), 62.09(s), 28.44(s), 28.17(s), 21.31(s), 15.78(s), 15.41(s), 14.06( s).
Embodiment 3
[0025] Synthesis of 1-(4-methoxyphenyl)-4-((4-methoxyphenyl)amino)-2-methyl-5-oxo-2,5-dihydro-1H- Ethyl pyrrole-2-carboxylate
[0026]
[0027] In this example, the aniline in Example 1 was replaced with equimolar p-methoxyaniline, and the other steps were the same as in Example 1 to obtain solid 1-(4-methoxyphenyl)-4-((4-methoxyphenyl) Oxyphenyl)amino)-2-methyl-5-oxo-2,5-dihydro-1H-pyrrole-2-carboxylic acid ethyl ester, the yield is 96%, and the structural characterization data are: 1 H NMR (600MHz, CDCl 3 )δ7.22-7.18 (m, 2H), 6.96 (d, J = 8.8Hz, 2H), 6.87 (d, J = 8.8Hz, 2H), 6.82 (d, J = 8.8Hz, 2H), 6.40 ( s,1H),5.76(s,1H),4.17-4.05(m,2H),3.74(d,J=14.5Hz,6H),1.54(s,3H),1.18(t,J=7.1Hz,3H ); 13 C NMR (151MHz, CDCl 3 )δ170.59(s),166.71(s),157.63(s),153.69(s),133.47(s),127.51(s),127.12(s),117.70(s),113.72(s),113.43( s), 104.79(s), 67.86(s), 60.99(s), 54.59(s), 54.43(s), 20.44(s), 13.06(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com