Assisted initiator amine-containing polymerizable thioxanthone carbazole visible light photoinitiator and preparation method thereof
A thioxanthone carbazole-based, visible light technology, applied in organic chemistry and other directions, can solve the problems of easy migration of fragments, odor toxicity, poor compatibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The product of the present invention 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate, which The structural formula is:
[0050]
[0051] Preparation of product 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate of the present invention method, the detailed steps are as follows:
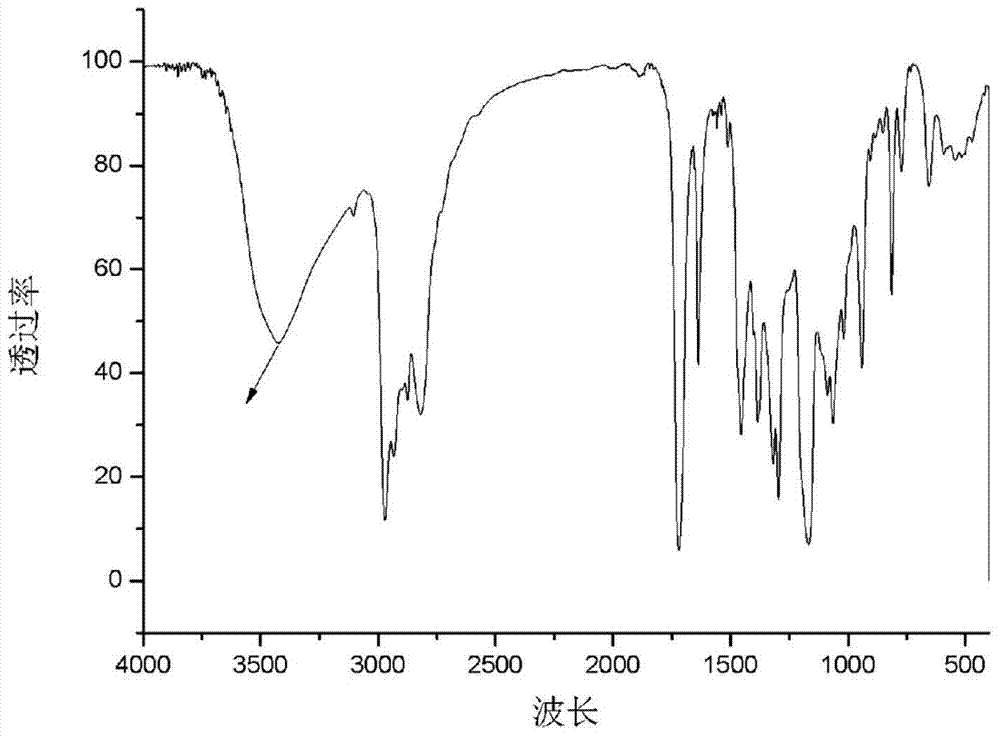
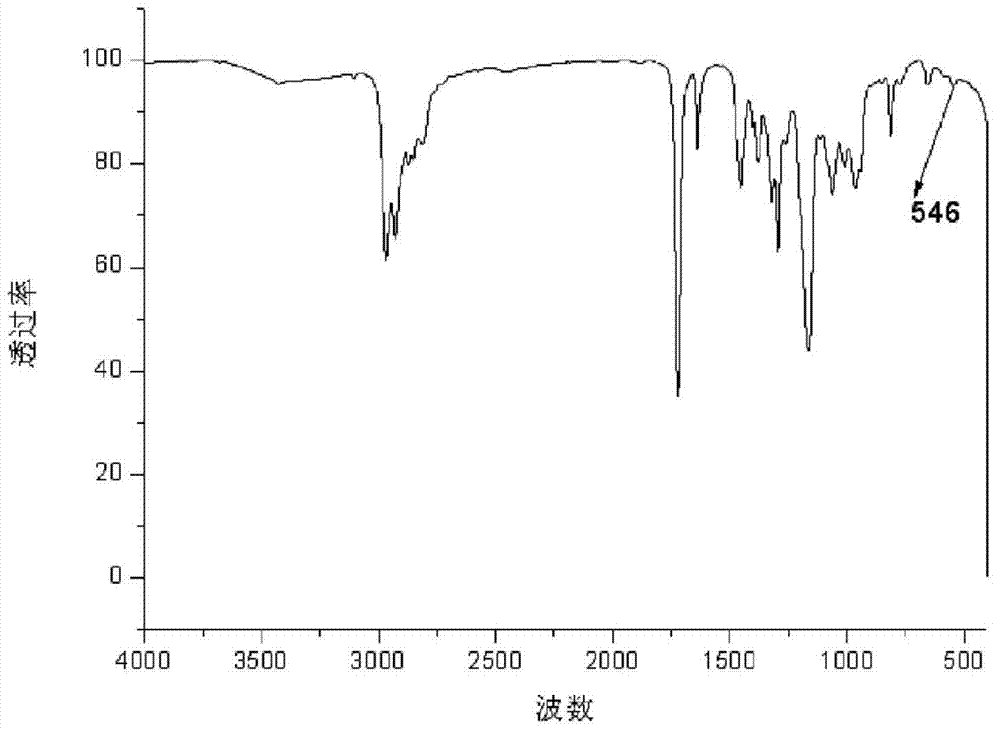
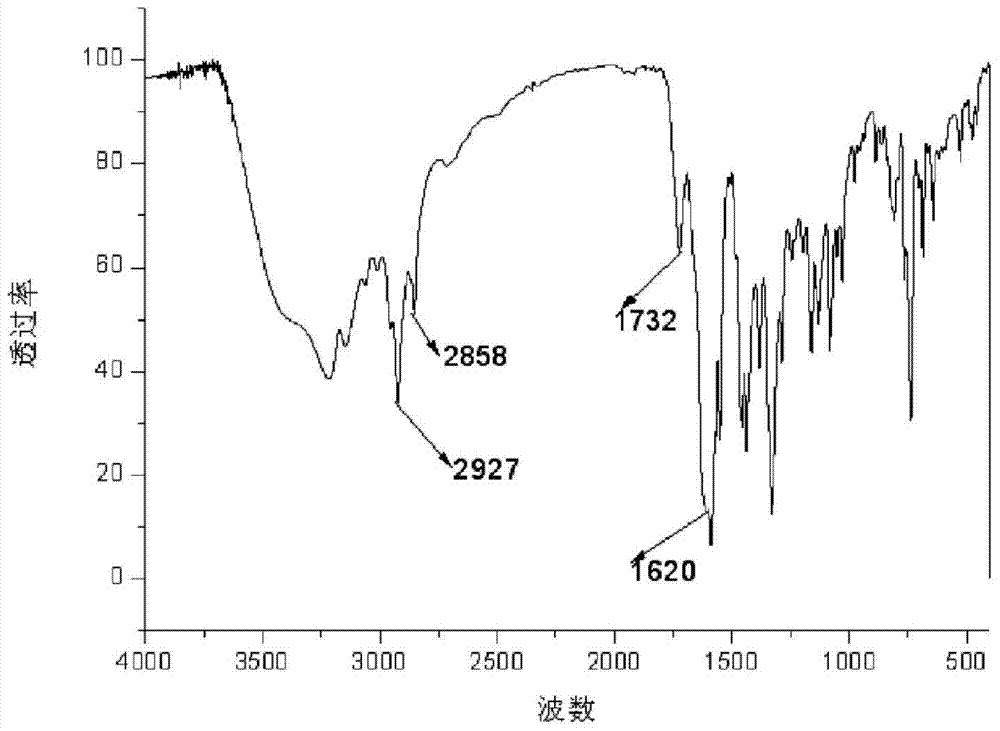
[0052] a. Put 29.24g (0.4mol) of diethylamine into a 100ml reaction flask, start stirring, heat to 56°C, slowly add 28.40g (0.2mol) of glycidyl methacrylate (GMA) into the reaction flask, and react 2h; warming up to 80°C, reacting for 3h; after the reaction, the resulting reactant was distilled to remove residual diethylamine to obtain 42.80g 3-(diethylamino)-2-hydroxypropyl methacrylate ( GMA-DEA) (see the attached infrared spectrum figure 1 );
[0053] b. Add 4.30g (0.02mol) of GMA-DEA and 0.79g (0.01mol) of pyridine into a 100ml reaction flask containing 30ml (44.50g) of chloroform at 1 to 3°C, and then dropwise a...
Embodiment 2
[0057] Embodiment 2: basically the same as Embodiment 1, the difference is:
[0058] Preparation of product 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate of the present invention method, the detailed steps are as follows:
[0059] a. Put 29.24g (0.4mol) of diethylamine into a 100ml reaction bottle, start stirring, heat to 40°C, slowly add 28.40g (0.2mol) of GMA into the reaction bottle, react for 1h; raise the temperature to 70°C for 4h ; After the reaction finished, the gained reactant was distilled to remove remaining diethylamine to obtain 42.08g GMA-DEA;
[0060] b. Add 4.30g (0.02mol) GMA-DEA into a 100ml reaction flask containing 40ml (59.20g) chloroform at 1-3°C, then add 5.40g (0.02mol) PBr dropwise 3 and a mixed solution of 10ml (14.80g) chloroform, reacted at 1-3°C for 1h; raised the temperature to 70°C for 4h;
[0061]After the reaction finished, the gained reactant was cooled to 20°C, added in 15ml of distilled water, then ...
Embodiment 3
[0064] Embodiment 3: basically the same as Embodiment 1, the difference is:
[0065] Preparation of product 3-(diethylamino)-2-(13-oxothiochromeno[2,3-B]carbazol-7-(13H)-yl)propyl methacrylate of the present invention method, the detailed steps are as follows:
[0066] a. Put 14.63g (0.2mol) of diethylamine into a 100ml reaction flask, start stirring, and heat to 60°C; slowly add 28.40g (0.2mol) of GMA into the reaction flask at this temperature, and react for 4 hours; heat up to Reaction at 70°C for 3 hours; after the reaction, the obtained reactant was distilled to remove residual diethylamine to obtain 38.95 g of GMA-DEA;
[0067] b. Add 4.30g (0.02mol) GMA-DEA and 1.58g (0.02mol) pyridine into a 100ml reaction flask containing 50ml (74.00g) chloroform at 1-3°C, then add 16.20g (0.06 mol)PBr 3 and a mixed solution of 15ml (22.20g) of chloroform, reacted at 1-3°C for 2h; raised the temperature to 40°C for 5h;
[0068] After the reaction was finished, the gained reactant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com