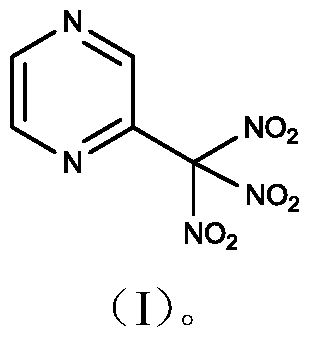
2-Trinitromethylpyrazine compound
A technology of trinitromethylpyrazine and compounds, which is applied in the directions of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry, etc., can solve the problems of low energy, low oxygen balance, low density and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
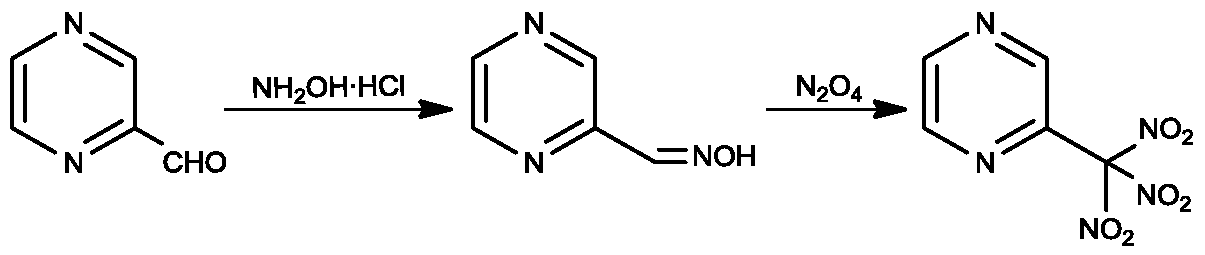
[0021] (1) Synthesis of 2-formaldehyde oxime pyrazine
[0022] Add 0.77g (11mmol) of hydroxylamine hydrochloride, 1.65g (11mmol) of sodium acetate and 60ml of ethanol into a 250ml three-necked flask, add 1.08g (10mmol) of 2-formaldehyde pyrazine with stirring, raise the temperature to 60°C, and react for 2h. After the reaction was completed, cool to room temperature, filter, and evaporate the filtrate to dryness. The obtained solid was washed with 100 ml of water, extracted 3 times with 50 ml of ethyl acetate, and the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain 1.12 g of a brown solid. was 91.1%.
[0023] Structure Identification:
[0024] NMR spectrum: 1 H NMR (600MHz, DMSO): δ12.04(s, 1H), 9.00(d, 1H), 8.65(dd, 1H), 8.61(d, 1H), 8.14(s, 1H); 13 C NMR (151MHz): δ147.76, 146.94, 144.31, 141.75
[0025] Elemental analysis: Molecular formula C 3 h 5 N 3 o
[0026] Theoretical values: C 48.78, H 4.0...
Embodiment 2
[0040] (1) Synthesis of 2-formaldehyde oxime pyrazine
[0041] Add 1.40g (20mmol) of hydroxylamine hydrochloride, 3.00g (20mmol) of sodium acetate and 60ml of ethanol into a 250ml three-necked flask, add 1.08g (10mmol) of 2-formaldehyde pyrazine under stirring, raise the temperature to 50°C, and react for 2h. After the reaction was completed, cool to room temperature, filter, and evaporate the filtrate to dryness. The obtained solid was washed with 100 ml of water, extracted 3 times with 50 ml of ethyl acetate, and the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain 1.19 g of a brown solid. was 96.8%.
[0042] Structure Identification:
[0043] NMR spectrum: 1 H NMR (600MHz, DMSO): δ12.04(s, 1H), 9.00(d, 1H), 8.65(dd, 1H), 8.61(d, 1H), 8.14(s, 1H); 13 C NMR (151MHz): δ147.76, 146.94, 144.31, 141.75
[0044] Elemental analysis: Molecular formula C 3 h 5 N 3 o
[0045] Theoretical values: C 48.78, H 4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com