Immobilized enzyme reactor based on trimethylolpropane trimethacrylate monolithic column
A technology of trimethylolpropane trimethacrylate and immobilized enzyme, which is applied in the directions of enzyme production/bioreactor, bioreactor/fermenter for specific purposes, bioreactor/fermenter combination, etc. Simple, stable and reproducible, efficient and rapid enzymatic hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
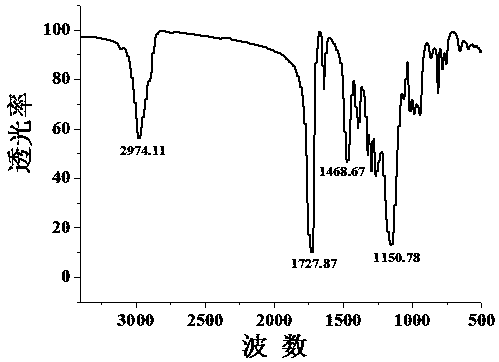
[0018] Preparation of organic monolithic column: 200 μL of TRIM, 680 μL of isooctane and 120 μL of toluene as porogen, 3.6 mg of azobisisobutyronitrile as initiator, mixed with ultrasound (150 W, 10 min) to form a pre-polymerization solution Inject into the treated capillary, seal both ends of the capillary with rubber stoppers, place in a 50°C water bath, take out the capillary after reacting for 24 h, and rinse with acetonitrile to remove unreacted solution and initiator.
[0019] Reduction of trypsin disulfide bond: use 20 mM Tris-HCl buffer (pH 8.0) to prepare trypsin solution with a concentration of 10 mg / mL and tris(2-carbonylethyl) phosphate hydrochloride with a concentration of 1 mg / mL (TCEP) solution. Then the two solutions were mixed according to the ratio of TCEP:trypsin solution at 1:10 (v / v), and the mixed solution was reacted at 25°C for 3 h to reduce and break the disulfide bond in trypsin. Then the mixture was centrifuged at 4°C (10000 rpm) for 10 min to remov...
Embodiment 2
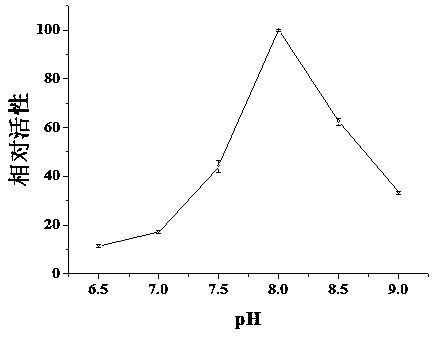
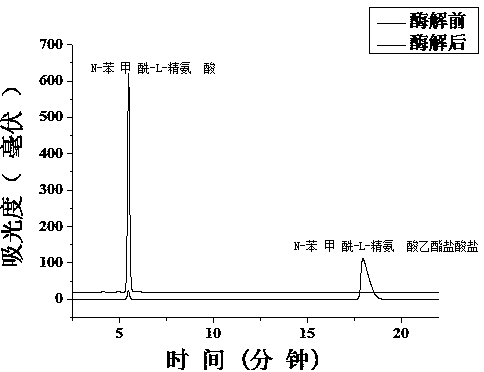
[0022] Enzyme activity assay: Trypsin activity assay is achieved by enzymatically hydrolyzing BAEE into BA. Using 5-25mM BAEE (in 20 mM Tris-HCl buffer, pH 8.0) as the substrate, it was digested by the same monolithic column under the same conditions, and the flow rate was 1 μL / min. The enzymatic hydrolysis products were collected by EP tubes and detected by reversed-phase high performance liquid chromatography. Michaelis constant (K m ) and maximum response rate (V max ) is calculated by the Mie equation. Use the Linewaver-Burk plot to plot 1 / V versus 1 / [S]:
[0023]
[0024] A straight line can be obtained. The intercept of the straight line on the horizontal axis is -1 / Km, and the vertical intercept is 1 / V max , can find K m with V max .
[0025] The detection conditions of high performance liquid chromatography are as follows:
[0026] Mobile phase: 80mM KH 2 PO 4 (pH 3.87):methanol=7:3
[0027] Flow rate: 1ml / min
[0028] Column temperature: 25°C
[0029]...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap