Method for producing acrylic acid from glycerin
A technology of acrylic acid and glycerol, applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., can solve the problems of high equipment requirements, high cost of ionic liquid catalysts, and large investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
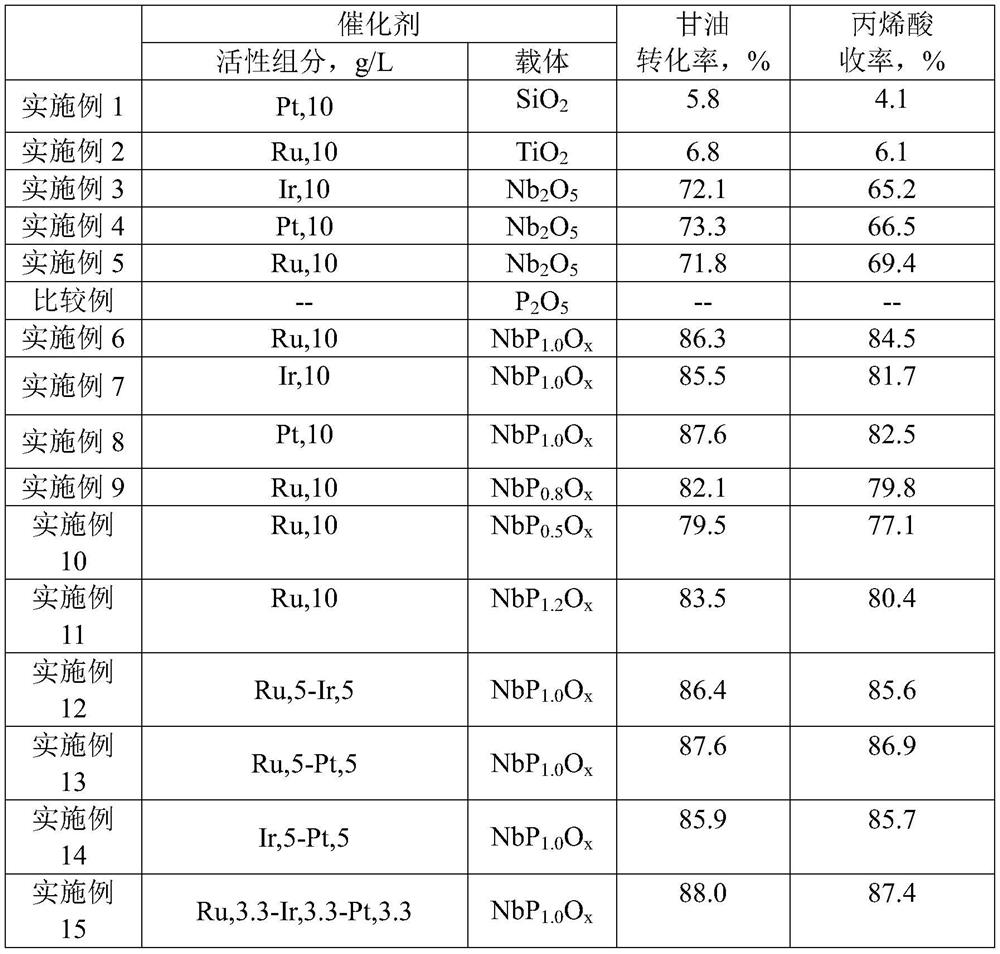
[0066] Take 400ml of commercially available SiO 2 Carrier powder, 20g graphite, 40g deionized water, mixed evenly, pelletized, dried at 120°C, calcined at 500°C for 2 hours, prepared Raschig ring carrier particles.
[0067] 3.5g platinum tetrachloride (PtCl 4 ) was dissolved in 100g of water to obtain a platinum tetrachloride aqueous solution. Take 200ml of carrier particles, pour the platinum tetrachloride aqueous solution into it, stir thoroughly, dry at 120°C, and roast at 500°C for 2 hours to obtain a catalyst. The catalytic activity is shown in Table 1 .
Embodiment 2
[0069] Take 400ml of commercially available TiO 2 Carrier powder, 20g graphite, 40g deionized water, mixed evenly, pelletized, dried at 120°C, calcined at 500°C for 2 hours, prepared Raschig ring carrier particles.
[0070] 5.2g hydrated ruthenium trichloride (RuCl 3 ·3H 2 O) dissolved in 100g of water to obtain an aqueous solution of ruthenium trichloride, take 200ml of carrier particles, pour the aqueous solution of ruthenium trichloride into it, fully stir, dry at 120°C, and roast at 500°C for 2 hours to obtain a catalyst. The catalytic activity is shown in the table 1.
Embodiment 3
[0072] Take 400ml of commercially available niobium pentoxide carrier powder, 20g of graphite, and 40g of deionized water, mix them uniformly, punch into tablets, dry at 120°C, and roast at 500°C for 2 hours to prepare Raschig ring carrier particles.
[0073] 3.6g iridium trichloride hydrate (IrCl 3 ·3H 2 O) be dissolved in 100g of water to obtain iridium trichloride aqueous solution, take 200ml of carrier particles, pour the iridium trichloride aqueous solution into it, fully stir, dry at 120°C, and roast at 500°C for 2 hours to obtain the catalyst. The catalytic activity is shown in the table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com