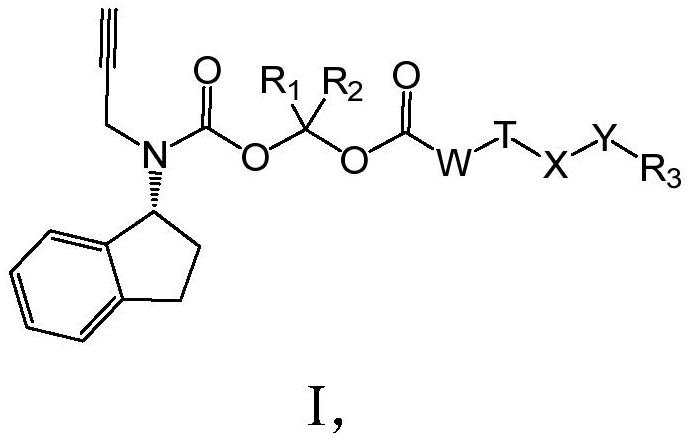
A long-acting rasagiline prodrug and its preparation method and application
A prodrug and drug technology, applied in the field of long-acting rasagiline prodrug and its preparation, can solve the problems of low melting point, forgetfulness, poor memory, etc., and achieve the effects of low solubility, prolonged release speed and high melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
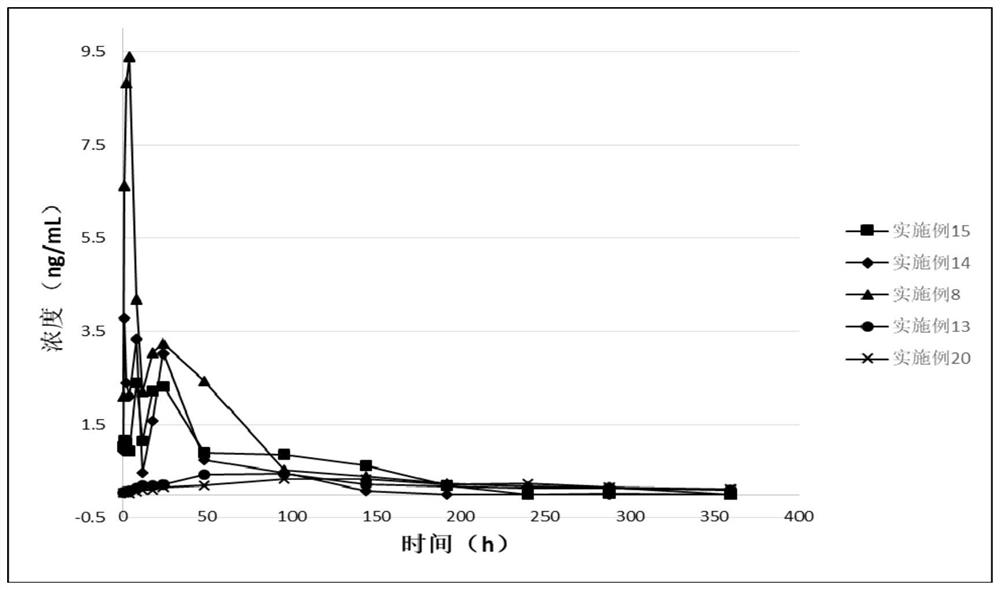
Image
Examples
Embodiment 1
[0086] Example 1 (Preparation of ((rasagiline-N-formyl)oxy)-methyl palmitate (see International Patent Application WO2013088255)
[0087]
[0088] Dissolve 1.0 g of rasagiline-N-carbonyl chloride methyl ester and 0.3 g of TBAI in THF / DMF (5+4 mL), add 1.5 g of potassium palmitate, and heat overnight at an external temperature of 57°C under argon protection. Stop the reaction, spin off THF, add isopropyl ether 30mL and stir for 10min, filter, and wash the filtrate with water (15mL filtrate) successively, saturated NaHCO 3 Washed, dried over anhydrous sodium sulfate, concentrated, and the concentrate was subjected to silica gel column chromatography (PE / EA=20:1-10:1) to obtain 0.76 g of a viscous product, yield: 41.53%.
[0089] 1 H-NMR (CDCl 3 ,400MHz)δ7.26(m,2H),7.19(m,2H),5.87(m,2.5H),5.78(m,0.5H),4.17(d,J=14.0Hz,0.5H),4.02( d,J=14.0Hz,0.5H),3.62(d,J=14.0Hz,0.5H),3.52(d,J=14.0Hz,0.5H),3.06(m,1H),2.86(m,1H) ,2.47(m,1H),2.36(m,2H),2.24(m,1H),2.21(s,0.5H),2.15(s,0.5H),1.6...
Embodiment 2
[0091] The preparation of embodiment 2 ((rasagiline-N-formyl) oxygen group)-naphthalene acetate methyl ester
[0092]
[0093] Dissolve 0.9 g of rasagiline-N-carbonyl chloride methyl ester and 0.25 g of TBAI in 15 mL of acetonitrile, add 0.92 g of potassium naphthalene acetate, and heat for 5 h at an external temperature of 60°C under the protection of argon. The reaction was stopped, the solvent was evaporated by rotary evaporation, 20 mL of ethyl acetate was added to dissolve, and saturated NaHCO 3 Wash (10mL×3), wash with saturated NaCl (10mL), anhydrous NaCl 2 SO 4 Dried, filtered, and spin-dried to obtain 1.38 g of crude product as light brown syrup. The crude product was recrystallized from methanol to obtain 0.65 g of pure rasagiline-N-carbomethyl naphthalene acetate as a white solid powder.
[0094] 1 HNMR (CDCl 3,400MHz)δ8.01(dd,J=14.8,8.4Hz,1H),7.84(m,2H),7.5(m,4H),7.22(m,3H),7.09(m,1H),5.90(m ,1.5H),5.82(dd,J=14.8,6.0Hz1H),5.54(t,J=8.0Hz,0.5H),4.16(s,1.07H)...
Embodiment 3
[0096] Example 3 (Preparation of ((rasagiline-N-formyl)oxy)-methyl stearate (see International Patent Application WO2013088255)
[0097]
[0098] Dissolve 2.0 g of rasagiline methyl carbochloride and 0.56 g of TBAI in 40 mL of DMF, add 3.2 g of potassium stearate, and heat the reaction overnight at an external temperature of 65° C. under the protection of argon. Stop the reaction, add 80mL of water, add 80mL of isopropyl ether for extraction, then use saturated NaHCO 3 Wash (30mL×2), saturated NaCl wash (30mL), anhydrous NaCl 2 SO 4 Dry, filter and spin dry to obtain 4.32g of crude product. The crude product was subjected to silica gel column chromatography (PE / EA=10:1) to obtain 3 g of the product as light yellow syrup with a yield of 51.55%.
[0099] 1 HNMR (CDCl 3 ,400MHz)δ7.25(m,2H),7.19(m,2H),5.87(m,2.5H),5.66(m,0.5H),4.12(d,J=17.6Hz,0.5H),3.98( d,J=17.6Hz,0.5H),3.57(d,J=17.6Hz,0.5H),3.47(d,J=17.6Hz,0.5H),3.06(m,1H),2.85(m,1H) ,2.46(m,1H),2.37(m,2H),2.24(m,1H),2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com