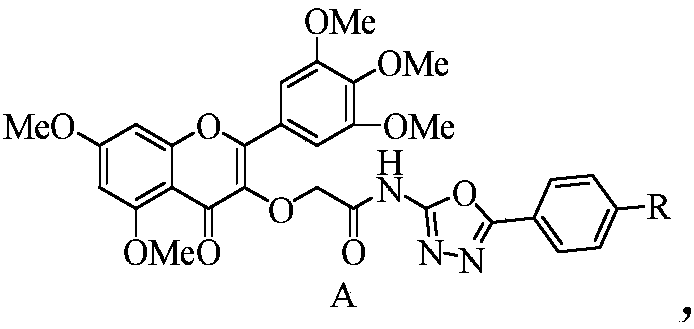
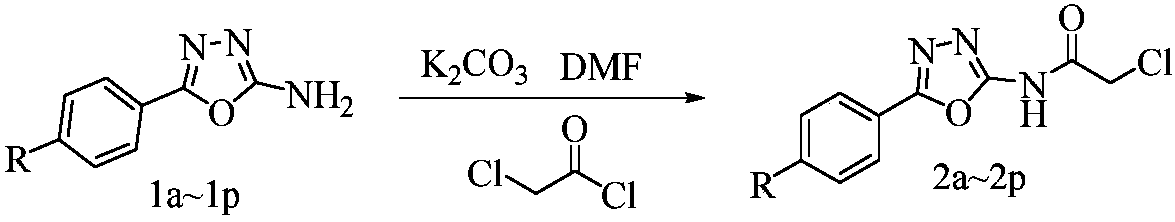
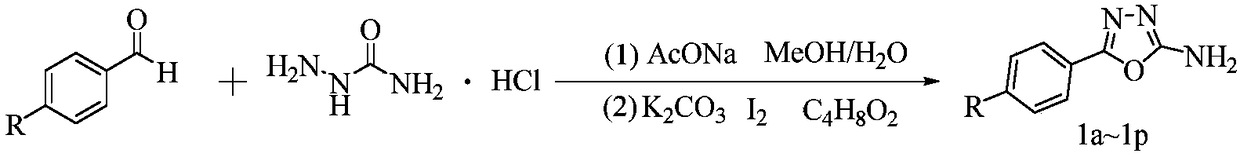Myricetin derivative containing amide-oxadiazole and preparation method and application of myricetin derivative
A technology of oxadiazole and derivatives, applied in the field of preparation of myricetin derivatives, can solve the problem of less applied research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 2-((5,7-dimethoxy-4-one-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy-N-(5- The preparation method of phenyl-1,3,4-oxadiazol-2-yl)acetamide (target compound 4a), comprises the following steps:
[0029] (1) Preparation of 2-amino-5-phenyl-1,3,4-oxadiazole
[0030] Add semicarbazide hydrochloride (9.42mmol) and sodium acetate (9.42mmol) to a 100mL round-bottomed flask, dissolve them with 20mL of secondary water, then slowly add 20mL of methanol containing benzaldehyde (9.42mmol) dropwise, at room temperature Stir for 20 minutes. Spin the solvent to dryness, add 40mL 1,4-dioxane, potassium carbonate (28.37mmol), iodine element (10.37mmol), heat at 80-85°C for 4-6 hours, track the reaction by TLC until no more Variety. The reactant was poured into 25% aqueous sodium thiosulfate solution, extracted with a mixed solvent of dichloromethane:methanol=10:1, dried with anhydrous sodium sulfate, spin-dried, N,N-dimethylformamide and Recrystallization from dichloromethane gave ...
Embodiment 2
[0038] 2-((5,7-dimethoxy-4-one-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy-N-((5 -(4-methyl)phenyl)-1,3,4-oxadiazol-2-yl)acetamide (target compound 4b) preparation method, comprising the following steps:
[0039] (1) Preparation of 2-amino-5-((4-methyl)-phenyl)-1,3,4-oxadiazole
[0040] As in the (1) step of embodiment 1, the difference is that p-tolualdehyde is used as a raw material.
[0041] (2) Preparation of 2-chloro-N-((5-(4-methyl)phenyl)-1,3,4-oxadiazole)-acetamide
[0042] As in step (2) of Example 1, the difference is that 2-amino-5-((4-methyl)-phenyl)-1,3,4-oxadiazole is used as the raw material.
[0043] (3) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0044] As in embodiment 1 (3) step.
[0045] (4) 2-((5,7-dimethoxy-4-one-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy-N- Preparation of ((5-(4-methyl)phenyl)-1,3,4-oxadiazol-2-yl)acetamide.
[0046] As in step (5) of Example 1, the difference is that 2-chloro-N-((5-(4-methyl)phenyl)-1,3,4-...
Embodiment 3
[0048] 2-((5,7-dimethoxy-4-one-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy-N-((5 -(4-methoxy)phenyl)-1,3,4-oxadiazol-2-yl)acetamide (target compound 4c) preparation method, comprising the following steps:
[0049] (1) Preparation of 2-amino-5-((4-methoxy)-phenyl)-1,3,4-oxadiazole
[0050] As in the first (1) step of embodiment 1, the difference is that p-methoxybenzaldehyde is used as a raw material.
[0051] (2) Preparation of 2-chloro-N-((5-(4-methoxy)phenyl)-1,3,4-oxadiazole)-acetamide
[0052] As in step (2) of Example 1, the difference is that 2-amino-5-((4-methoxy)-phenyl)-1,3,4-oxadiazole is used as the raw material.
[0053] (3) Preparation of 3-hydroxy-3',4',5',5,7-pentamethoxymyricetin
[0054] As in embodiment 1 (3) step.
[0055] (4) 2-((5,7-dimethoxy-4-one-2-(3,4,5-trimethoxyphenyl)-4H-chromen-3-yl)oxy-N- Preparation of ((5-(4-methoxy)phenyl)-1,3,4-oxadiazol-2-yl)acetamide.
[0056] As in step (4) of Example 1, the difference is that 2-chloro-N-((5-(4-met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com