Cleavable polymer drug conjugates
A technology of drug conjugates and polymers, applied in the field of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] Example 1: Preparation of a Glycine-Phenylalanine-Leucine-Glycine (GFLG) Cleavable Linker with Methacrylate Functionality
[0209] The GFGL-MA linker was prepared according to the literature method described in K. Ulbrich et al., Journal of Controlled Release, 64, 2000, 63-79.
Embodiment 2
[0210]Embodiment 2: the preparation of docetaxel-GFGL-methacrylate (MA) monomer
[0211] According to the literature method described in Ghandehari et al., Mol. Pharm., 2011, 8(4), 1090-1099, docetaxel (DTX) was coupled to the GFGL-MA linker to obtain DTX-GFLG-MA.
[0212] Docetaxel (0.335 g, 4.1 mmol), 4-(dimethylamino)pyridine (DMAP, 0.049 g, 4.0 mmol) and MA-GFLG-OH (0.188 g, 4.0 mmol) were dried under vacuum. The reaction mixture was dissolved in anhydrous N,N-dimethylformamide (DMF, 5 mL) under nitrogen, cooled with an ice bath (salt / ice) at <0 °C, and diisopropylcarbadiene was added dropwise Imine (DIPC, 76 μL, 4.89 mmol).
[0213] The reaction mixture was then stirred for 1 hour, then the ice bath was removed, the mixture was allowed to warm to room temperature, stirred overnight, and was analyzed by thin layer chromatography (TLC, eluent dichloromethane (DCM):methanol (MeOH) (95:5 )) Progress is monitored for disappearance of starting material and formation of MA-GFL...
Embodiment 3
[0214] Example 3: Preparation of polymer-drug conjugates comprising docetaxel and PEG in the side chain
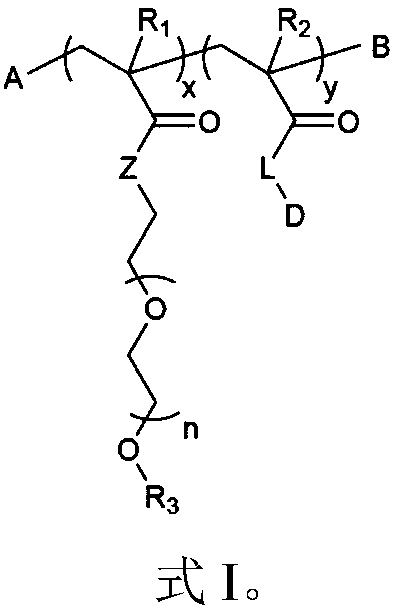
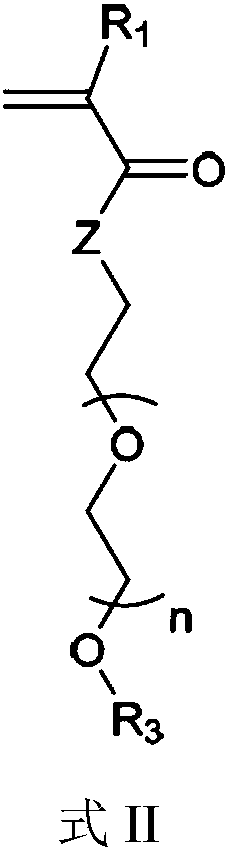
[0215] Polyethylene glycol methyl ether methacrylate (PEGMEMA) (Mn: 300) and DTX-GFGL-MA were polymerized in the presence of AIBN as an initiator and DMF as a solvent to obtain PEGMEMA-DTX.
[0216]
[0217] Scheme 1: Schematic representation of the copolymerization of PEGMEMA and DTX-GFLG-MA monomers.
[0218] In the above figure, (*) indicates the AIBN fragment as the terminal group of the polymer-drug conjugate.
[0219] By changing the ratio of PEGMEMA, PEGMEMA-DTX conjugates with different molecular weights can be obtained.
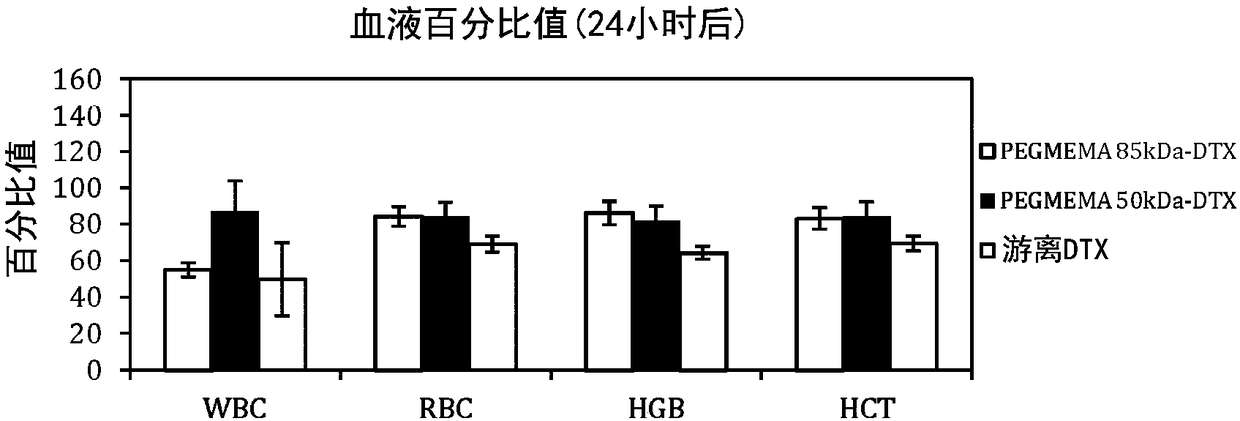
[0220] In order to demonstrate that the present invention is applicable to PEGMEMA-DTX conjugates of various molecular weights, the inventors prepared 50 kDa and 85 kDa PEGMEMA-DTX polymer-drug conjugates and performed pharmacokinetic tests on them.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com