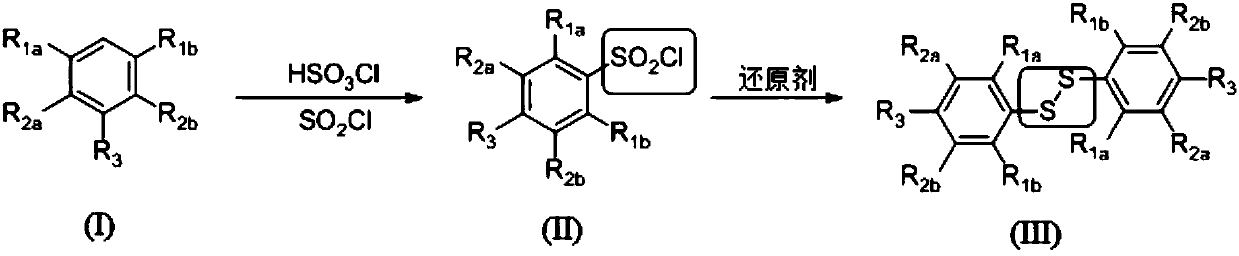
Preparation method for symmetric diaryl disulfide
A diaryl disulfide, symmetrical technology, applied in the field of preparation of symmetrical diaryl disulfide, can solve the problems of high cost, limited sources of halogenated hydrocarbons and thiophenols, and achieve short reaction time and cheap reagents The effect of easy availability, high application value and industrial production value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The following reaction schemes are provided as illustrations:
[0030]
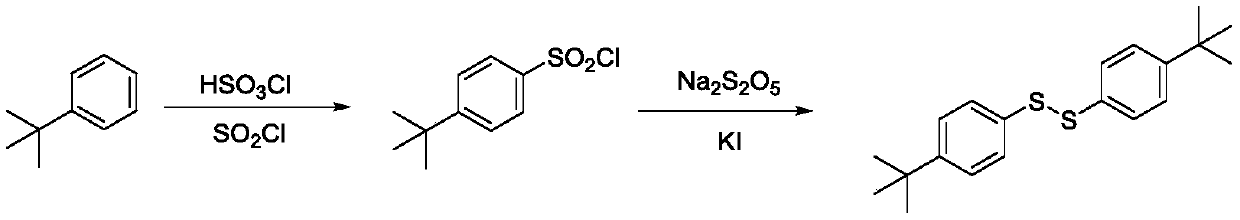
[0031] Add 33.55g of tert-butylbenzene and 80g of 1,2-dichloroethane into a 500ml four-neck flask equipped with a stirring rod, condenser, thermometer and constant pressure dropping funnel. After cooling down to 5°C, start adding dropwise Add 34.95g of chlorosulfonic acid for 1 hour. After the dropwise addition, continue to keep warm for 5 hours, then raise the temperature to 60°C, add 35.68g of thionyl chloride dropwise for 1 hour, keep warm for 5 hours, and then distill under reduced pressure Remove 1,2-dichloroethane to obtain p-tert-butylbenzenesulfonyl chloride, which can be directly used to prepare disulfide;
[0032] Add the 39g p-tert-butylbenzenesulfonyl chloride prepared above, 3.34g potassium iodide, 150g glacial acetic acid in a 500ml four-necked flask with a stirring rod, a condenser tube, a thermometer and a constant pressure dropping funnel, and heat up to 85°C with The solid fee...
Embodiment 2
[0034] The following reaction schemes are provided as illustrations:
[0035]
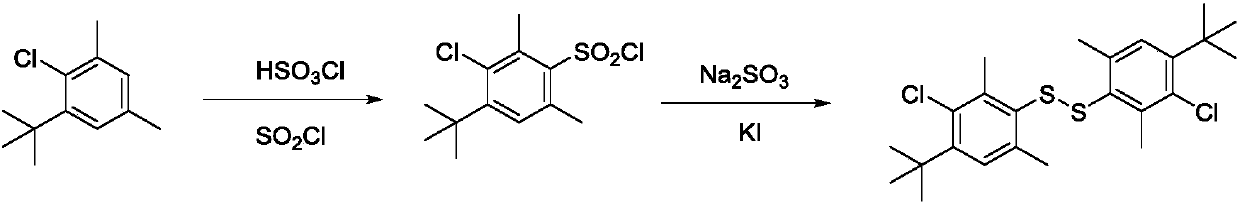
[0036] Add 22.00 g of 2,6-dimethyl-4-tert-butylbenzene, 80 g of cyclohexane in a 500 ml four-necked flask with a stirring rod, condenser, thermometer and constant pressure dropping funnel, and cool to 5 After ℃, start to add 15.64g of chlorosulfonic acid dropwise for 1h. After the dropwise addition, continue the heat preservation reaction for 5h, then raise the temperature to 80°C and add 15.91g of thionyl chloride dropwise for 1h. 5h, followed by distillation under reduced pressure to remove cyclohexane to obtain 3-chloro-2,6-dimethyl-4-tert-butylbenzenesulfonyl chloride, which can be directly used to prepare disulfide;
[0037] In the 500ml four-necked flask that has stirring bar, condenser tube, thermometer and constant pressure dropping funnel, add above-mentioned prepared 2,6-dimethyl-4-tert-butylbenzenesulfonyl chloride 33g, potassium iodide 2.23g, 150g of glacial acetic acid, heat up to ...
Embodiment 3
[0039] The following reaction schemes are provided as illustrations:
[0040]
[0041] Add 19.53g of benzene and 80g of ethyl acetate into a 500ml four-necked flask equipped with a stirring rod, condenser, thermometer and constant pressure dropping funnel. The duration is 1h. After the dropwise addition, continue to keep warm for 5h, heat up to 65°C, add 35.68g of thionyl chloride dropwise, add dropwise for 1h, keep warm for 5h, and then distill off ethyl acetate under reduced pressure to obtain benzenesulfonate Acyl chlorides can be directly used to prepare disulfides;
[0042] Add 12.9g of benzenesulfonyl chloride and 150g of glacial acetic acid prepared above into a 500ml four-necked flask equipped with a stirring rod, condenser, thermometer and constant pressure dropping funnel, raise the temperature to 80°C, and slowly add 6.05g of phosphorous acid dropwise , added dropwise for 1 hour, after 1 hour of heat preservation reaction, slowly pour the reaction solution into ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap