Nucleic acid combination for detecting respiratory virus and application thereof
A respiratory and viral technology, applied in the field of nucleic acid combination detection of respiratory viruses, can solve the problems of difficult to distinguish clinical manifestations, achieve rapid and objective detection results, high sensitivity, and reduce pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
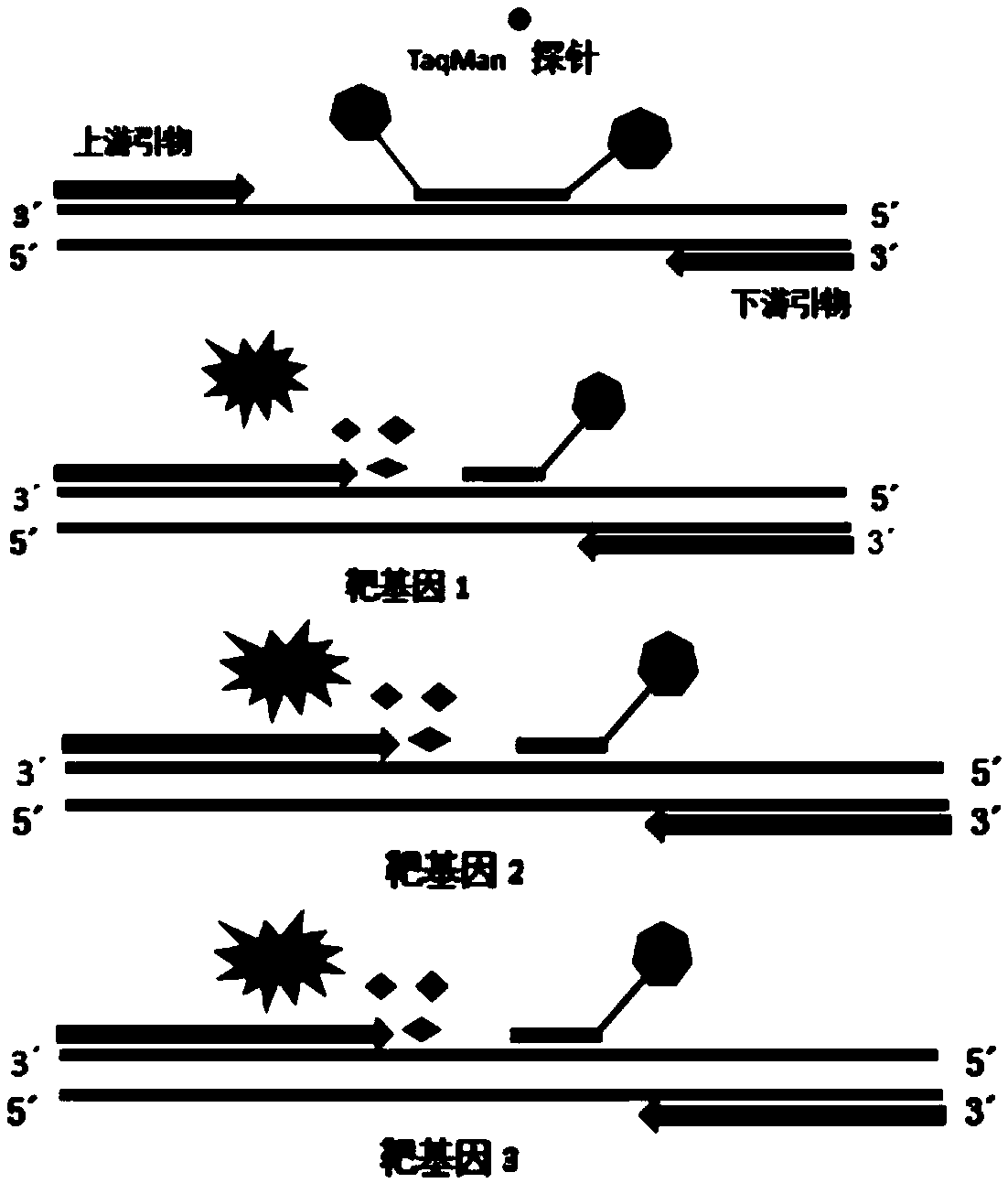
[0058] The invention provides a nucleic acid detection kit for quickly detecting type 4 herpes virus, adenovirus and respiratory syncytial virus:
[0059] Among them, the nucleic acid amplification reaction solution includes tris (50mM), potassium chloride (100mM), magnesium chloride (15mM), nucleotide mixture (dUTP and dTTP are 1.25mM, dATP, dCTP and The dGTP concentration is 2.5mM respectively).
[0060] The herpes virus type 4, adenovirus and respiratory syncytial virus reaction solution contains the following components:
[0061] Component (1): A pair of primers for detecting human herpes virus type 4, the base sequences are shown in SEQ ID No. 1 and SEQ ID No. 2, and the primer concentrations are both 600 nM (nmol / L), and the corresponding The base sequence of the probe is shown in SEQ ID No. 3, and the probe concentration of SEQ ID No. 3 is 400 nM (nmol / L); the 5'end of the probe is labeled with a fluorescent reporter group FAM, and the 3'end is labeled with Fluorescence quen...
Embodiment 2
[0084] Sensitivity test of the kit of the invention
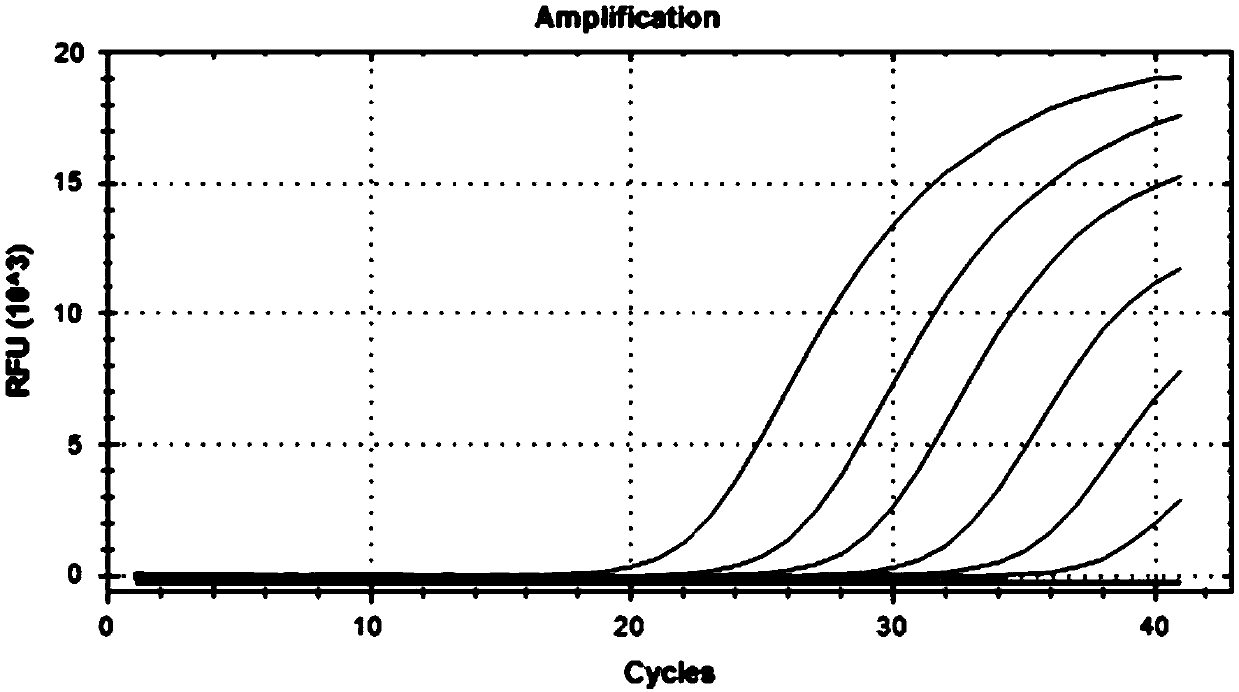
[0085] The positive reference is the inactivated type 4 herpes virus, adenovirus and respiratory syncytial virus culture medium. Will contain 1×10 4 The nucleic acid extract of TCID 50 / ml type 4 herpes virus, adenovirus and respiratory syncytial virus culture medium is diluted to 1×10 4 , 1×10 3 , 1×10 2 The sensitivity test was carried out at six concentrations of, 50, 10, and 1TCID50 / ml.
[0086] The negative reference materials were influenza A virus H1N1 subtype, parainfluenza virus type 1, metapneumovirus, and coronavirus culture medium.
[0087] The kit of the present invention (prepared in Example 1) was used for detection according to the same method as in Example 1. See the result Figure 3-5 . Figure 3-5 Among them, the kit provided by the present invention has good sensitivity, and the CT value changes gradually as the concentration decreases.
[0088] The test results show that the kit of the invention has high sensit...
Embodiment 3
[0090] Specificity test of kit detection of the present invention
[0091] Use the kit of the present invention (prepared in Example 1) to detect type 4 herpes virus, adenovirus and respiratory syncytial virus, influenza A virus H1N1 subtype, parainfluenza virus type 1, metapneumovirus, coronavirus, etc., according to the example 1 The same method is used for detection.
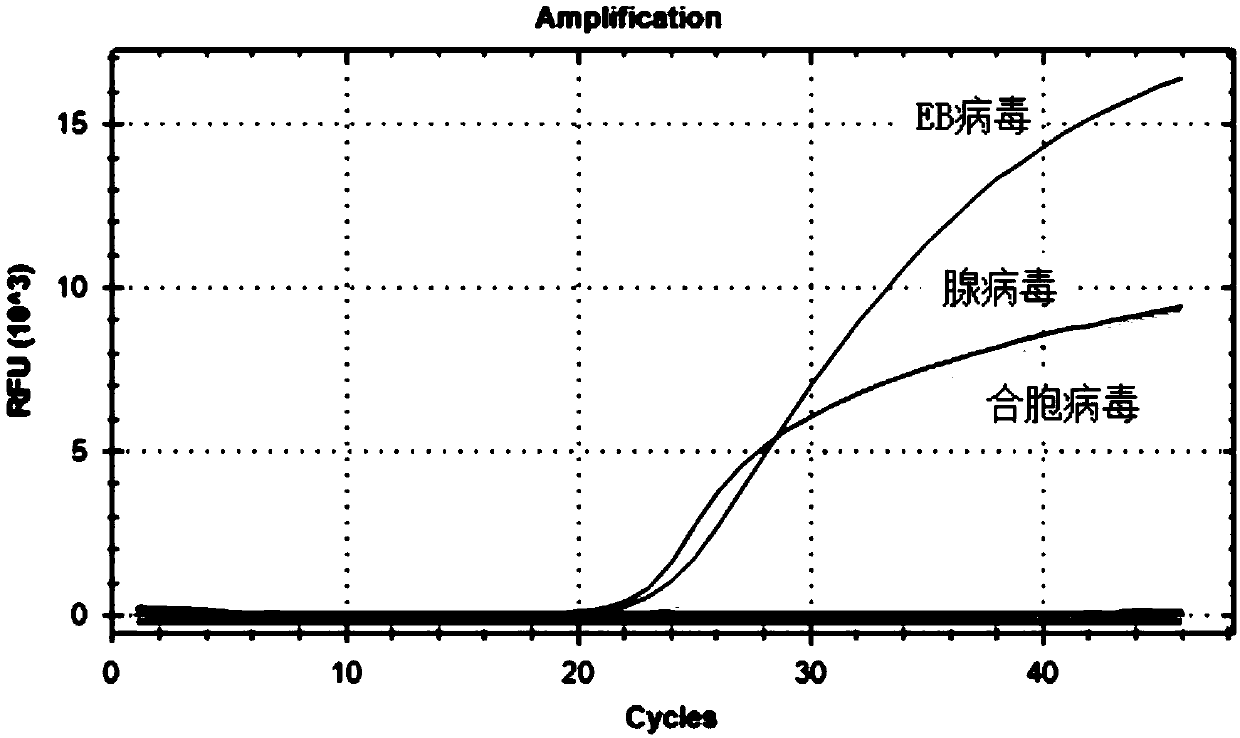
[0092] The test results showed that the FAM channel only amplified herpesvirus type 4, and the CY5 channel only amplified adenovirus. The HEX channel only amplifies the respiratory syncytial virus. The test results show that the detection kit of the present invention can specifically amplify type 4 herpes virus, adenovirus and respiratory syncytial virus without cross-reaction with other viral nucleic acids. The results are shown in Figure 6-8 .
[0093] Image 6 Among them, other viruses include adenovirus, respiratory syncytial virus, influenza A virus H1N1 subtype, parainfluenza virus type 1, metapneumovirus,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com