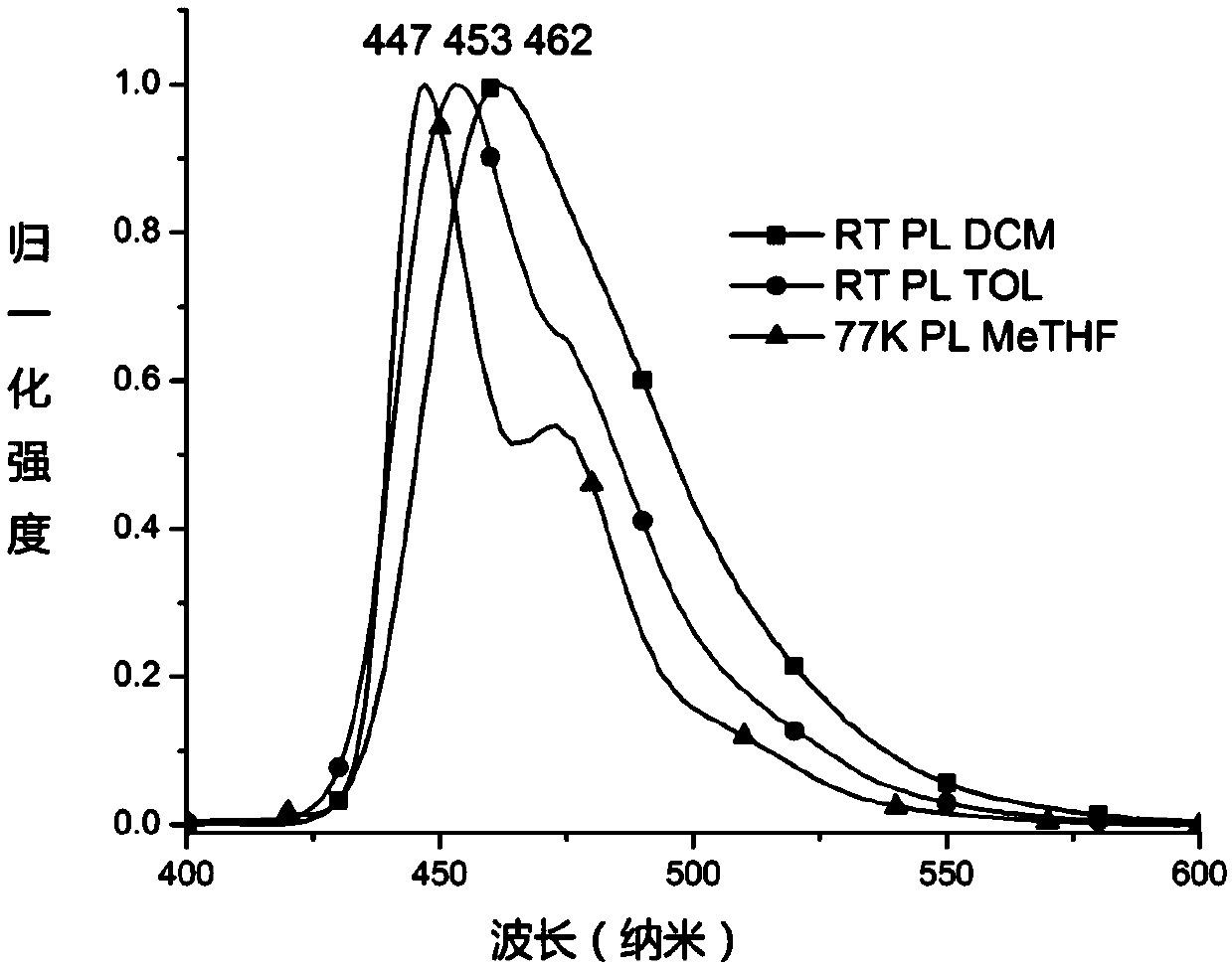
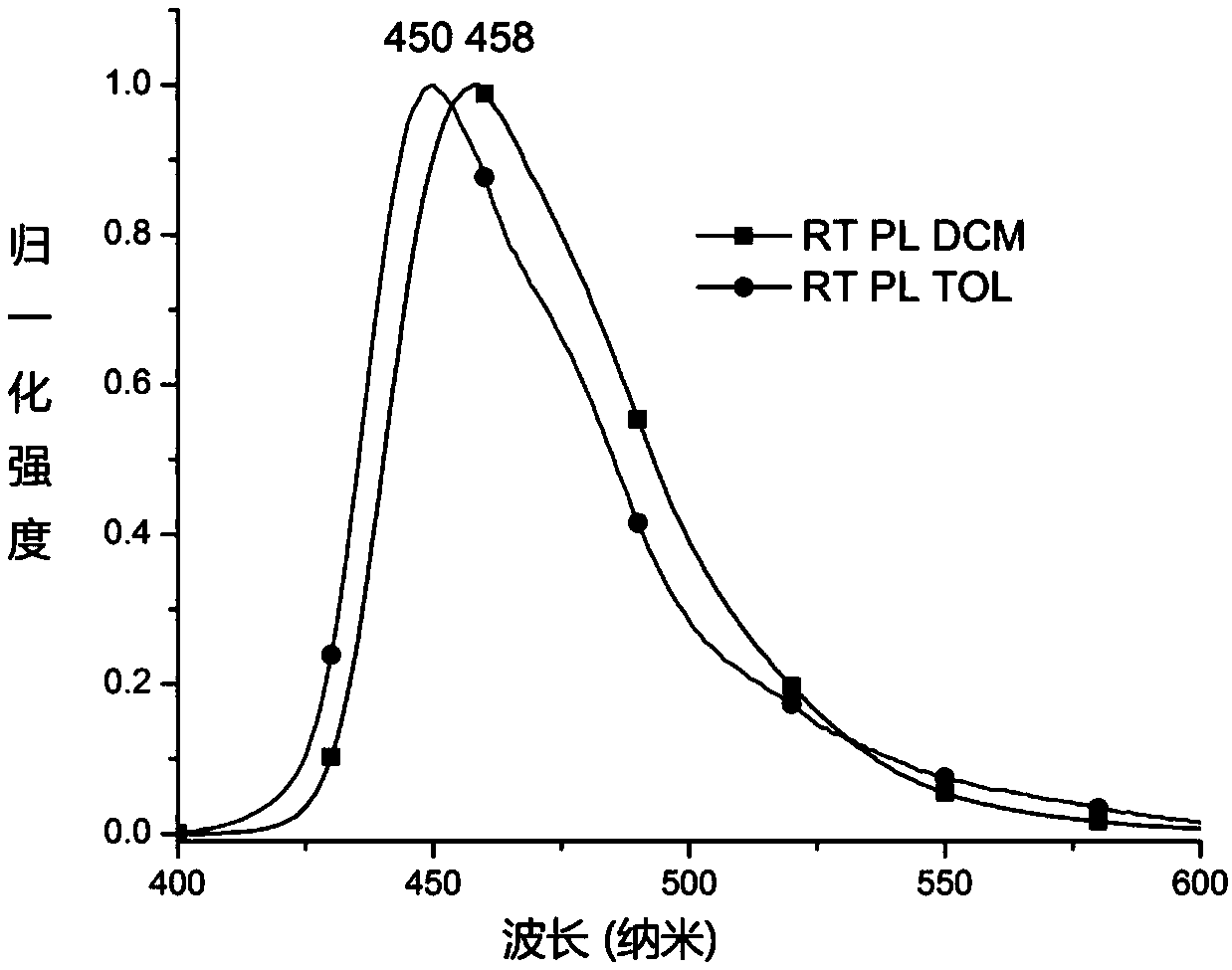
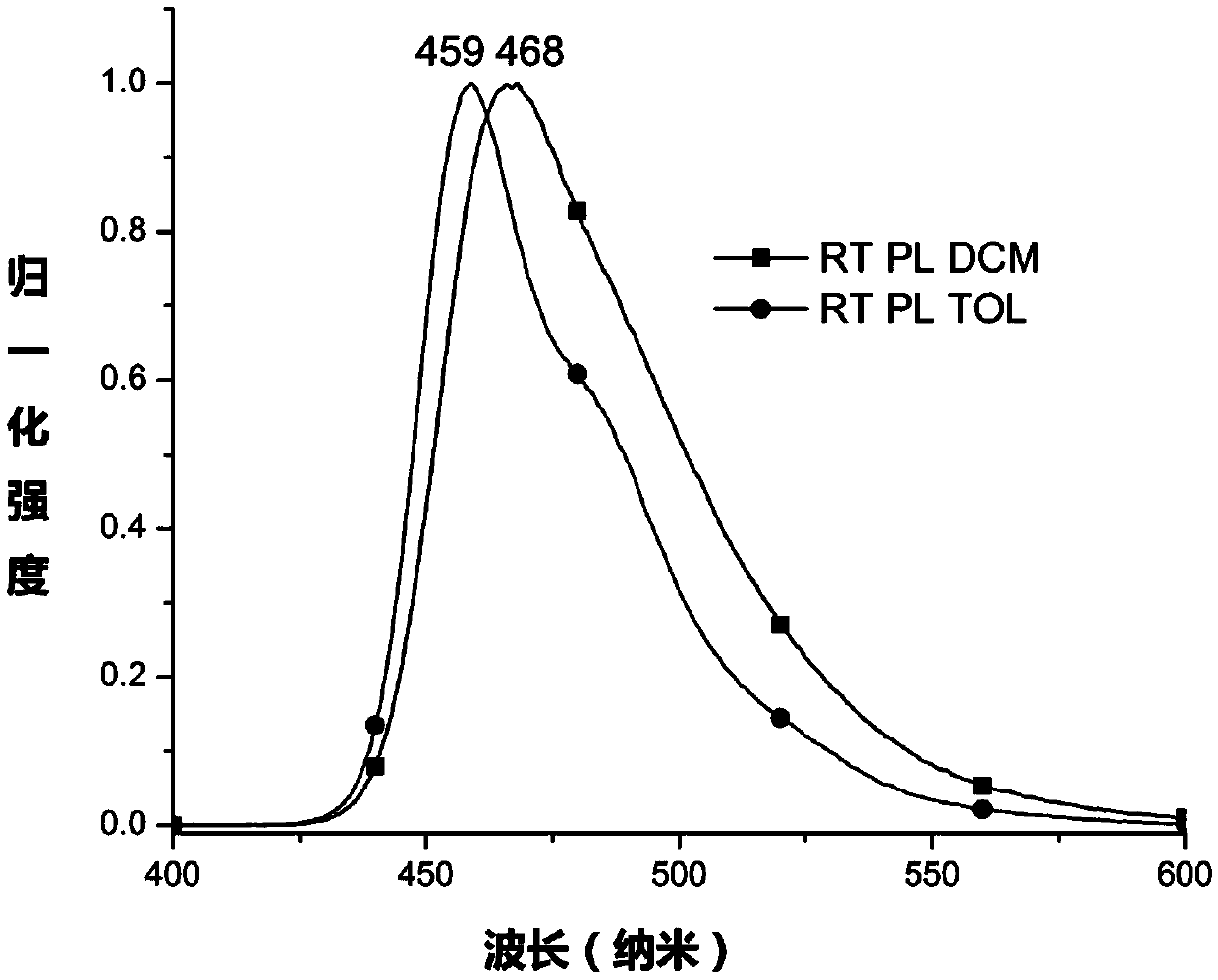
Pyrene compound containing benzo cyclohexyl alkane structure and organic light emitting device
A technology of benzocyclohexane and organic light-emitting devices, which is applied in the preparation of amino compounds from amines, light-emitting materials, organic chemistry, etc., can solve the problems of not meeting the requirements of long-life dark blue light-emitting compounds, and reduce the interaction , Improve luminous efficiency, increase the effect of steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Compound FBD-3
[0099]
[0100] Synthesis Step 1: Synthesis of Intermediate 1:
[0101]
[0102] Into a 75ml three-neck flask, sequentially add 30ml of toluene, 2.0g of 1-aminotetralin, 1.70g of 2,5-dimethylbromobenzene, 1.74g of sodium tert-butoxide, and 0.14g of tris(dibenzylideneacetone)palladium , 2-cyclohexylphosphine-2,6-methoxy-1,1-biphenyl 0.18g, after nitrogen replacement three times, the system was heated up to 85 degrees, and kept for 12 hours, the system was directly shrunk to dryness after cooling down, and the column layer Analysis, dichloromethane (DCM): petroleum ether (PE) = 1:10, 2.07 g of yellow solid was obtained. Yield 90%.
[0103] Synthesis Step 2: Synthesis of Compound FBD-3:
[0104]
[0105] Add 0.96 g of 1,6-dibromopyrene, 2.0 g of intermediate 3, 200 mg of palladium acetate, 5 ml of tri-tert-butylphosphine, 960 mg of sodium tert-butoxide, and 50 ml of toluene into a 100 ml three-necked flask. 85°C, heat preservation for 12h, the...
Embodiment 2
[0110] Compound FBD-O-5
[0111]
[0112] Synthesis Step 1: Synthesis of Intermediate 2
[0113]
[0114] Into a 75ml three-necked flask, add 30ml of toluene, 2.7g of 1-aminotetralin, 2.25g of 4-bromodibenzofuran, 2.2g of sodium tert-butoxide, 0.18g of tris(dibenzylideneacetone) palladium, 2 -Cyclohexylphosphine-2,6-methoxy-1,1-biphenyl 0.22g, after nitrogen replacement three times, the temperature of the system was raised to 85 degrees, and the reaction was kept for 12 hours, and the system was directly shrunk to dryness after cooling down, and column chromatography Dichloromethane (DCM): petroleum ether (PE) = 1:20 to obtain 2.28 g of a yellow solid. Yield 91%.
[0115] Synthesis Step 2: Synthesis of Compound FBD-O-5:
[0116]
[0117] Add 1.1g of 1,6-dibromopyrene, 2.2g of intermediate 2, 220mg of palladium acetate, 5ml of tri-tert-butylphosphine, 1.0g of sodium tert-butoxide, and 50ml of toluene into a 100ml three-necked flask. Heat at 85°C and keep warm for 1...
Embodiment 3
[0121] Compound FBD-P-1.
[0122]
[0123] Synthetic Step 1: Synthesis of Intermediate 3
[0124]
[0125] Into a 75ml three-necked flask, sequentially add 30ml of toluene, 2.7g of 1-aminotetralin, 2.12g of 3-bromobiphenyl, 2.2g of sodium tert-butoxide, 0.18g of tris(dibenzylideneacetone)palladium, 2-cyclo Hexylphosphine-2,6-methoxy-1,1-biphenyl 0.22g, nitrogen replacement three times, the system was heated up to 90 degrees, heat preservation reaction for 12 hours, the system was directly shrunk to dryness after cooling down, column chromatography, DCM: PE=1:10, 2.34 g of yellow solid was obtained. Yield 87%.
[0126] Synthesis Step 2: Synthesis of Compound FBD-P-1
[0127]
[0128] Add 1.1g of 1,6-dibromopyrene, 2.1g of intermediate 3, 220mg of palladium acetate, 5ml of tri-tert-butylphosphine, 1.0g of sodium tert-butoxide, and 50ml of toluene into a 100ml three-necked flask. Heat at 85°C and keep warm for 12 hours. After the system is concentrated, column chroma...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap