A kind of biological material semi-solid storage medium and its application
A biomaterial and semi-solid technology, applied in the field of biomedical materials, can solve the problems of long-term preservation of biofilms at room temperature, and achieve the effects of low cost, convenient use and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
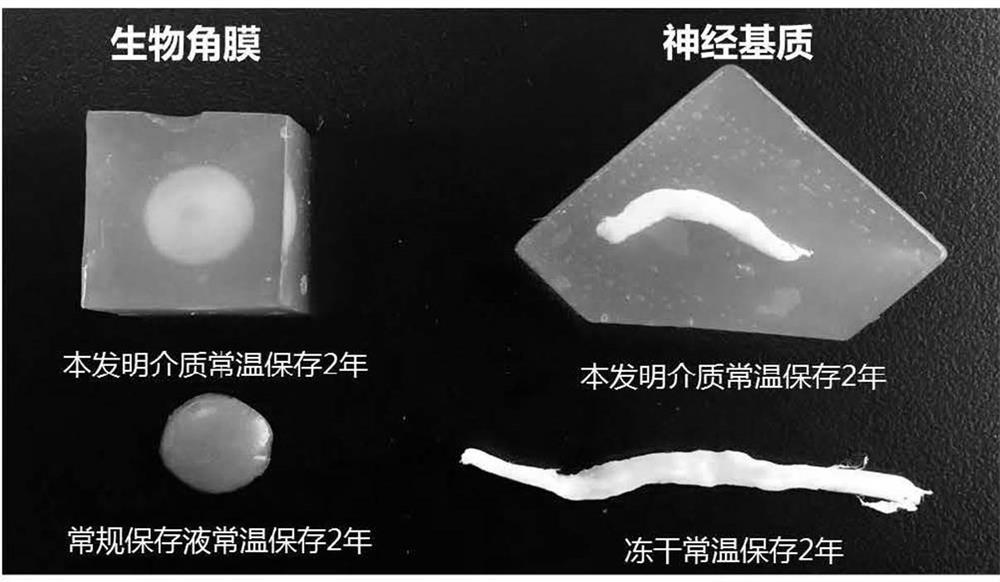
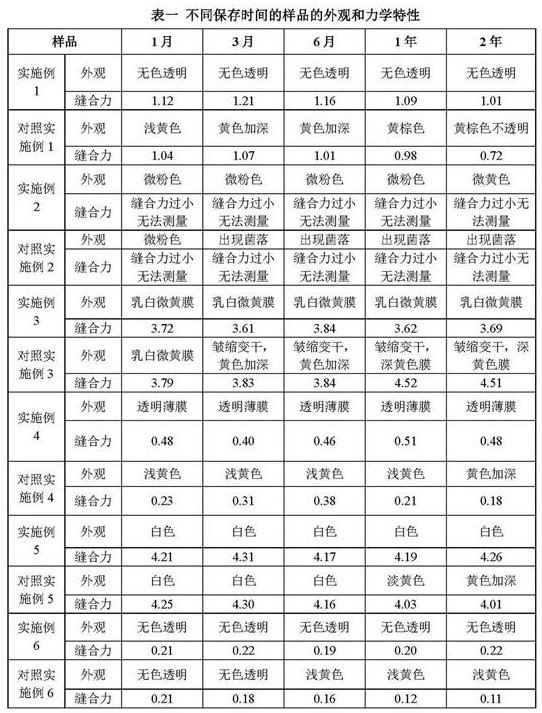
[0038] Weigh 2.0g low-melting point agarose (gel temperature 30-35 ℃) and 0.1g dextran (molecular weight 40kDa) and disperse in 80ml PBS buffer solution with a concentration of 0.2mol / L, heat and stir continuously until completely swollen, add 0.1g glycerin, stir evenly, dilute the solution to 100ml with the same buffer solution, make the final pH 7.2~7.4, divide into storage containers, cool to below 38 ℃, put the biological cornea sample into the solution to expand and remove Bubbles, sealed and transferred to 4°C to refrigerate until the whole becomes a stable semi-solid state, sterilized by irradiation, and can be stored at room temperature for 1 to 2 years for a long time.
Embodiment 2
[0040] Weigh 0.2g low-melting point agarose (gel temperature 20-28 ℃) and 1g hydroxypropylmethylcellulose (viscosity 30000mPa s) and disperse in 40ml neutral HEPES buffer solution with a concentration of 0.01mol / L, heat Stir continuously until it swells completely, add 40g of polyethylene glycol 550, stir evenly, dilute the solution to 100ml with the same buffer solution, make the final pH 7.2~7.4, divide into storage containers, cool to below 28 ℃, put Spinal cord extracellular matrix samples were placed in the solution, unfolded and air bubbles were removed, sealed and transferred to 4°C for refrigeration until the whole became a stable semi-solid, sterilized by irradiation, and stored at 4°C for 1 year.
Embodiment 3
[0042] Weigh 1.2g of low-melting point agarose (gel temperature ~25°C) and 0.4g of hyaluronic acid (molecular weight: 80kDa) and disperse in 50ml of neutral citrate buffer solution with a concentration of 0.05mol / L, heat and stir continuously until completely swollen , add 20g of propylene glycol, stir evenly, dilute the solution to 100ml with the same buffer solution, make the final pH 7.2~7.4, divide it into a storage container, and put the decellularized pericardium sample into the solution when it is cooled to about 30°C Inside, unfold and remove air bubbles, seal and transfer to 4°C to refrigerate until the whole becomes a stable semi-solid, sterilized by irradiation, and can be stored at room temperature for 1 to 2 years.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com