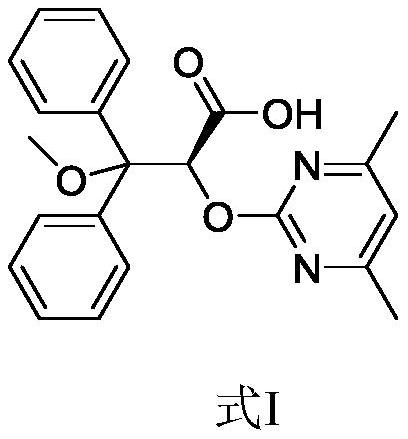
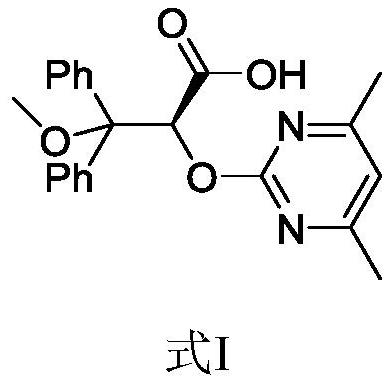
A kind of preparation method of ambrisentan
A compound and molar ratio technology, applied in the field of drug synthesis, can solve the problems of severe reaction conditions, influence of the quality and yield of the finished product of Ambrisentan, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 formula Va compound
[0049]
[0050] a) preparation of formula IIIa compound
[0051]
[0052] Add 3L of N,N-dimethylformamide into a 5L three-necked flask, add 300g of the compound of formula II, stir to dissolve; add 457g of anhydrous potassium carbonate, stir for 10min, then add 188g of benzyl bromide, heat to 30-35 The reaction was carried out under temperature control at ℃ for 2 h, followed by TLC until the spots of the compound of formula II disappeared, and the reaction solution of the compound of formula IIIa was directly put into the next reaction without treatment.
[0053] b) preparation of formula Va compound
[0054]
[0055] 256 g of the compound of formula IV were added in batches to the reaction solution of the compound of formula IIIa, stirred and heated to 90-100°C for 3 hours, followed by TLC monitoring until the compound of formula IIIa was completely reacted. The reaction product was extracted and separate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com