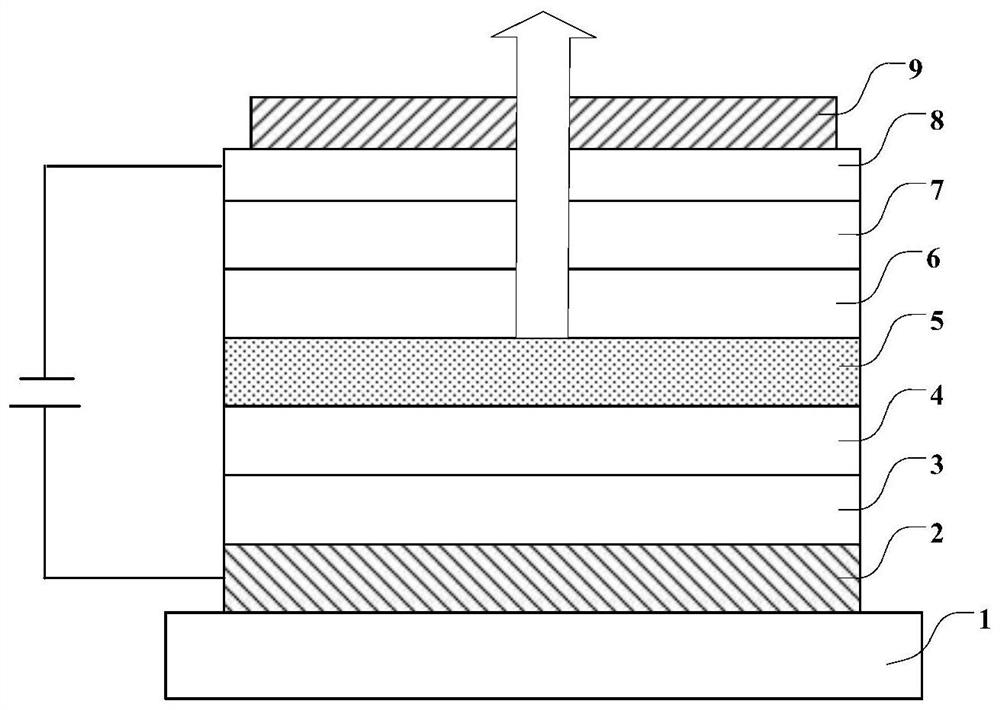
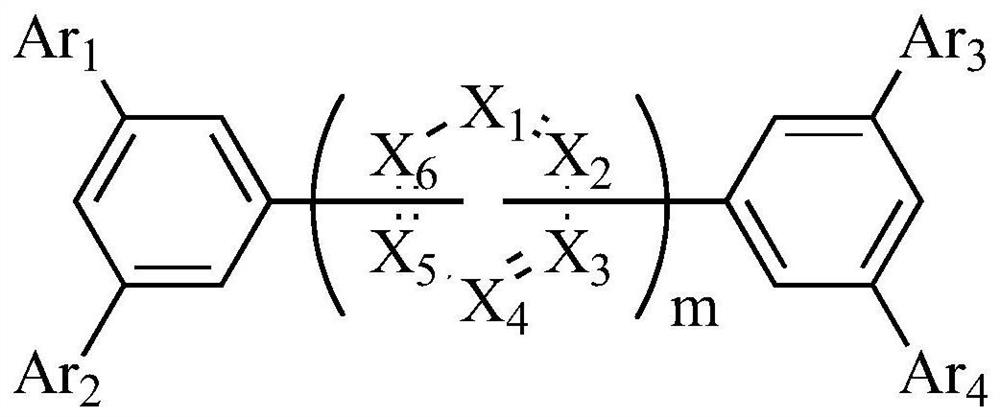Aromatic ring compound, display panel and display device
A technology for display panels and compounds, applied in organic chemistry, electrical solid devices, semiconductor devices, etc., can solve the problems of intermolecular close packing, low glass transition temperature, no solution to luminous efficiency, etc., and achieve short conjugation length. , High refractive index, the effect of improving light extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Synthesis of compound CP004
[0066]
[0067] In a 250ml round bottom flask, 3,5-dibromobiphenyl (15mmol) and potassium acetate (40mmol) were mixed with dry 1,4-dioxane (60mL), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and pinacol diboronate (25mmol) were mixed, and stirred at 90° C. under a nitrogen atmosphere for 48 hours. The resulting intermediate was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography. The product yielded intermediate CP004-1.
[0068] In a 250ml round bottom flask, CP004-1 (10mmol), 4-bromo-2-phenyl-[1,10]phenanthroline (12mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added to the mixture of toluene (30ml) / ethanol (20ml) and potassium carbonate (12mmol) aqueous solution (10ml), and the reaction was refluxed under nitrogen a...
Embodiment 2
[0073] Synthesis of compound CP017
[0074]
[0075] In a 250ml round bottom flask, mix 3,5-dibromobiphenyl (15mmol) and potassium acetate (40mmol) with dry 1,4-dioxane (60ml), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and pinacol diboronate (25mmol) were mixed, and stirred at 90° C. under a nitrogen atmosphere for 48 hours. The resulting intermediate was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography. The product yielded intermediate CP017-1.
[0076] In a 250ml round bottom flask, CP017-1 (10mmol), 2-bromo-[1,10]phenanthroline (12mmol) and Pd(PPh 3 ) 4(0.3mmol) was added to the mixture of toluene (30ml) / ethanol (20ml) and potassium carbonate (12mmol) aqueous solution (10ml), and the reaction was refluxed under nitrogen atmosphere for 12h...
Embodiment 3
[0081] Synthesis of Compound CP020
[0082]
[0083] In a 250ml round bottom flask, mix 3,5-dibromobiphenyl (15mmol) and potassium acetate (40mmol) with dry 1,4-dioxane (60ml), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and pinacol diboronate (25mmol) were mixed, and stirred at 90° C. under a nitrogen atmosphere for 48 hours. The resulting intermediate was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography. The product yielded intermediate CP020-1.
[0084] In a 250ml round bottom flask, CP020-1 (10mmol), 2-bromo-quinoline (12mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added to a mixture of toluene (30ml) / ethanol (20ml) and potassium carbonate (12mmol) aqueous solution (10ml), and the reaction was refluxed under nitrogen atmosphere for 12h. The resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com