Compositions and methods for selective protein expression
A protein structure, protein technology, applied in the field of compositions and methods for selective protein expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1136] Example 1: Materials and methods for Examples 1-13.
[1137] construct
[1138] The following constructs shown in Table 22 were used in Examples 1-13.
[1139] Note that the invention encompasses both orientations of heavy and light chains.
[1140] Table 22. Sequences of various constructs (signal peptide used in bold; furin cleavage site underlined)
[1141]
[1142]
[1143]
[1144]
[1145]
[1146]
[1147]
[1148]
[1149]
[1150]
[1151]
[1152]
[1153]
[1154]
[1155]
[1156]
[1157] general method
[1158] The following general materials and methods were used for the assays described in Examples 1-13.
[1159] CAR construct generation
[1160]The scFv used in the CAR construct evaluated in Example 3 was synthesized independently based on the anti-CD19 scFv sequence described above. The scFv was cloned into a CD8 hinge region together with a 4-1BB molecule and a CD3δ molecule in the heavy chain to li...
Embodiment 2
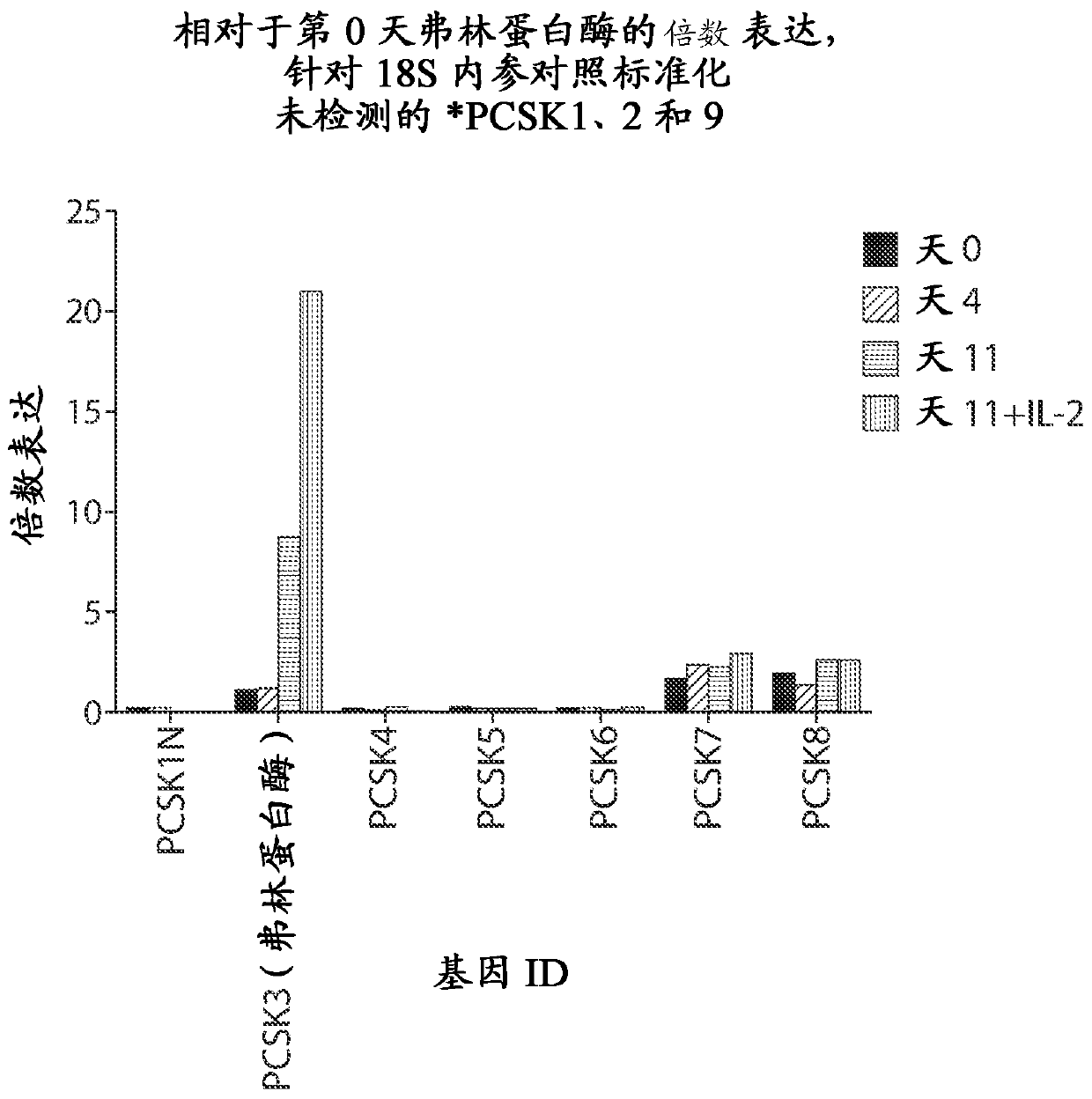
[1173] Example 2: Furin is the most highly expressed proprotein convertase in primary human T cells
[1174] Literature sources show that furin (FUR; PACE; PCSK3; SPC1 ) has a broad expression profile (Seidah et al., Nature Reviews Drug Discovery 11, 367-383 (May 2012)). Expression of proprotein convertase family members was analyzed by qRT-PCR. RNA was harvested from normal donor T cells on days 0, 4, and 11 after stimulation with anti-CD3 / anti-CD28 activation beads. During the culture period, an additional group was supplemented with 100 U / mL IL-2 and RNA was harvested on day 11. These data show that after activation with anti-CD3 / anti-CD28 beads, furin mRNA is more highly expressed than other members of the proprotein convertase family ( figure 1 ).
Embodiment 3
[1175] Example 3: CD19 CAR Expression Controlled by Compound Addition
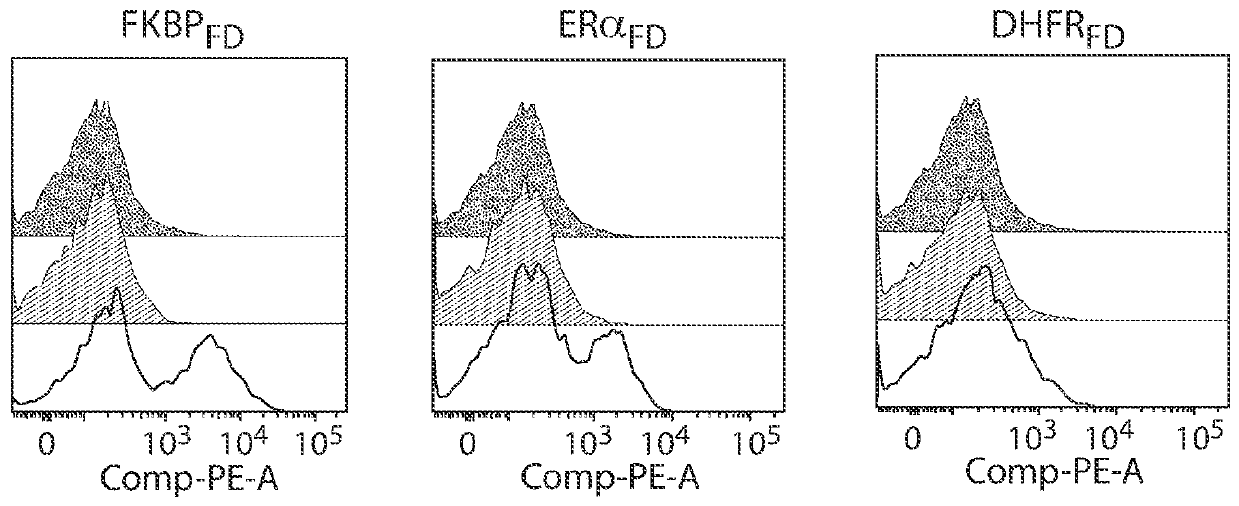
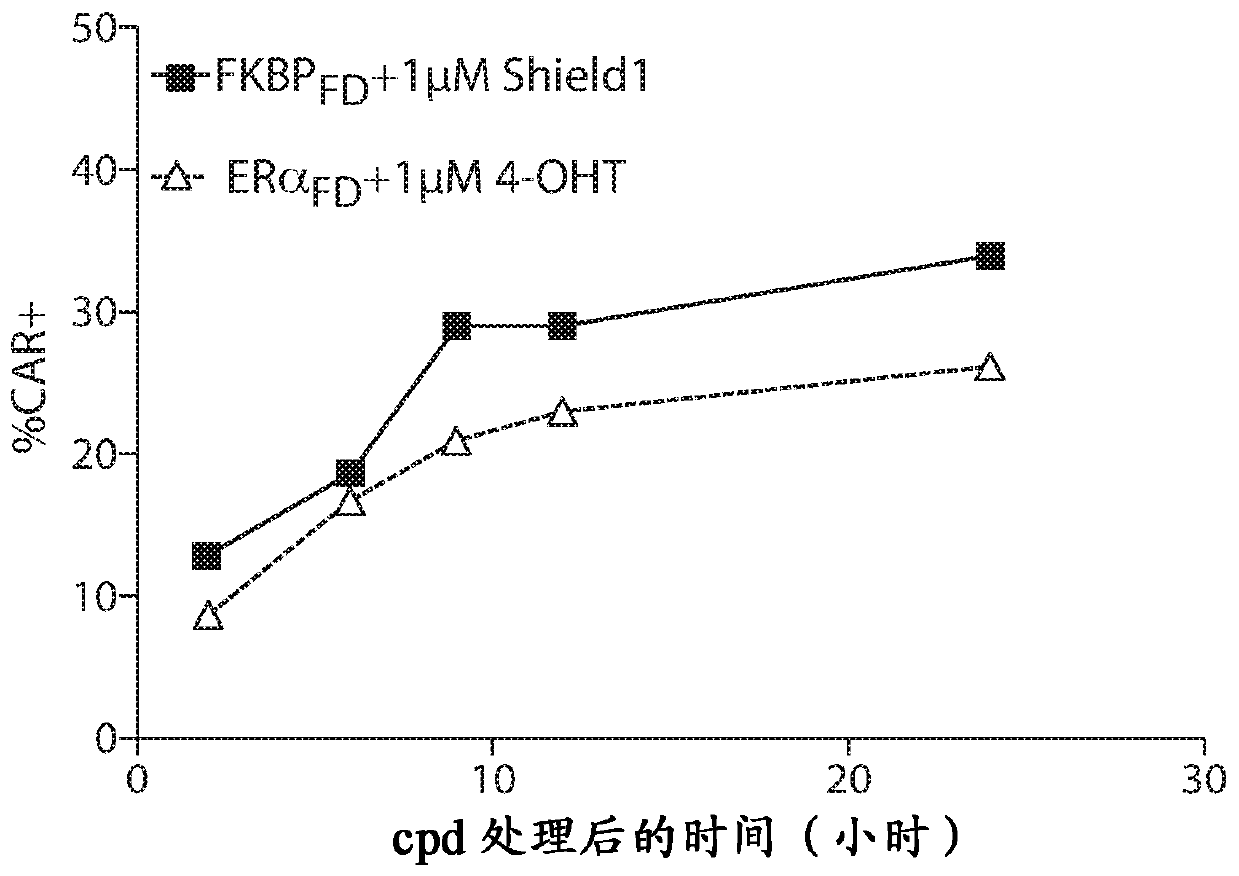
[1176] Furin degron domain (FKBP) fused to anti-CD19 scFv CAR construct in Jurkat T cells FD , ERα FD or DHFR FD ) transduction followed by treatment with the corresponding compound. FKBP were treated with 1 μM Shield1 FD transduced cells. ERα was treated with 1 μM bazedoxifene FD transduced cells. DHFR was treated with 1 mM TMP FD transduced cells. In the presence of the compound, the expression of anti-CD19 scFv was inducible ( figure 2 ). Black = UTD; gray = construct, no compound; white = construct, with compound. These data show that multiple degron domains lead to strict compound-dependent regulation of CAR 19 expression when combined with the furin cleavage domain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com