Caspofungin intermediate and synthesis method thereof
A synthesis method and compound technology, which are applied in the field of caspofungin intermediates and their synthesis, can solve the problems of increasing the cost of industrialization, low yield and stereoselectivity, polluting the environment, etc., so as to shorten reaction steps and reduce preparation and purification. The number of times, the effect of improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
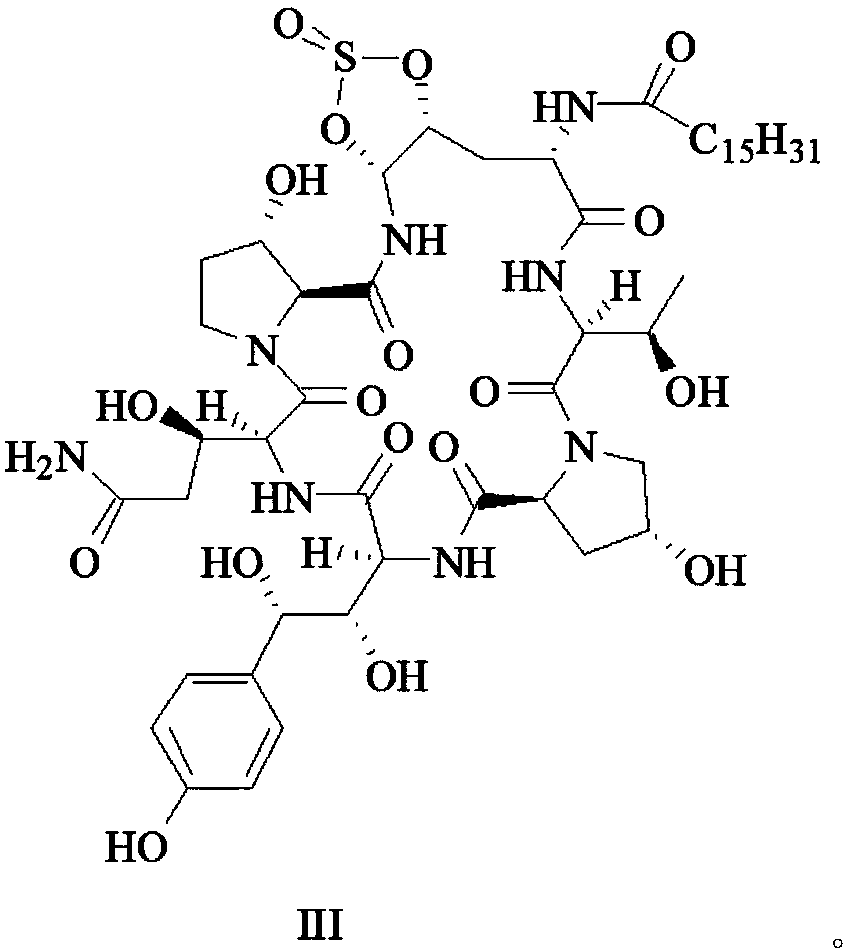
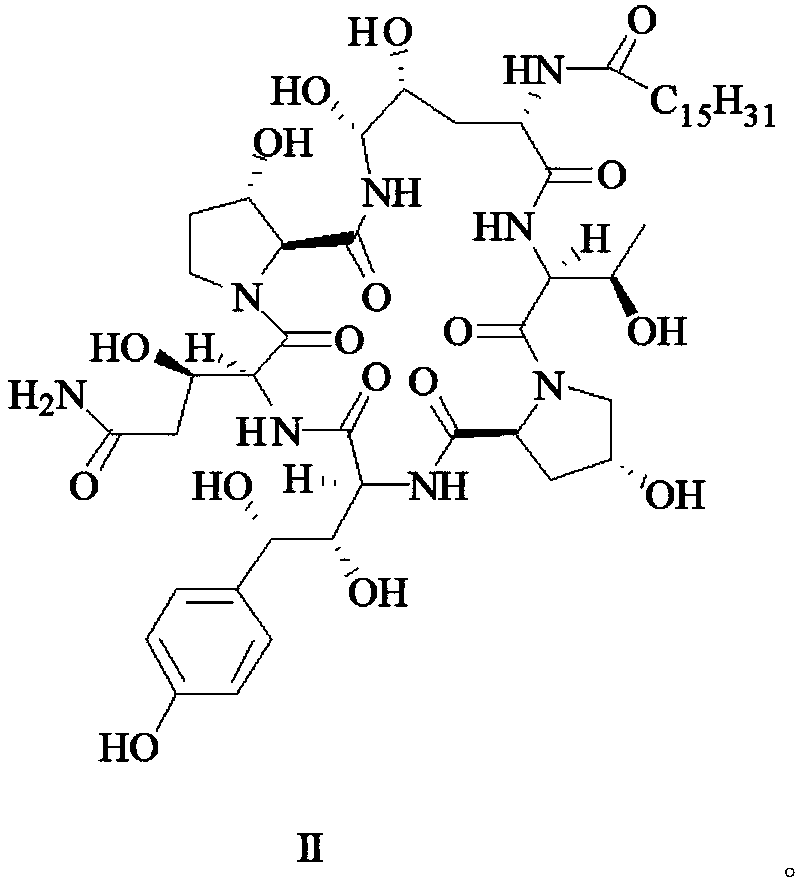
[0031] Formula II compound (10.00g, 9.4mmol) and chloroform (200mL) were placed in a 500mL three-neck flask, under nitrogen protection, placed in a low-temperature bath, and dropped to -30°C, and slowly added dropwise at this temperature Thionyl chloride ( SOCl 2 , 0.37g, 3.1mmol) in chloroform (20mL) solution, after the dropwise addition, the reaction was started, and the reaction process was followed and monitored by HPLC, and the reaction time was 1h. Add cold saturated potassium carbonate aqueous solution to the reaction solution until no more bubbles are generated, separate the organic layer, extract the aqueous layer with chloroform (50mL×2), combine the organic layers, wash with cold pure water until nearly neutral, and rinse with anhydrous sulfuric acid Sodium drying overnight, filtration, distillation under reduced pressure and vacuum drying gave the compound of formula III as a pale yellow solid. Yield 90.6%.
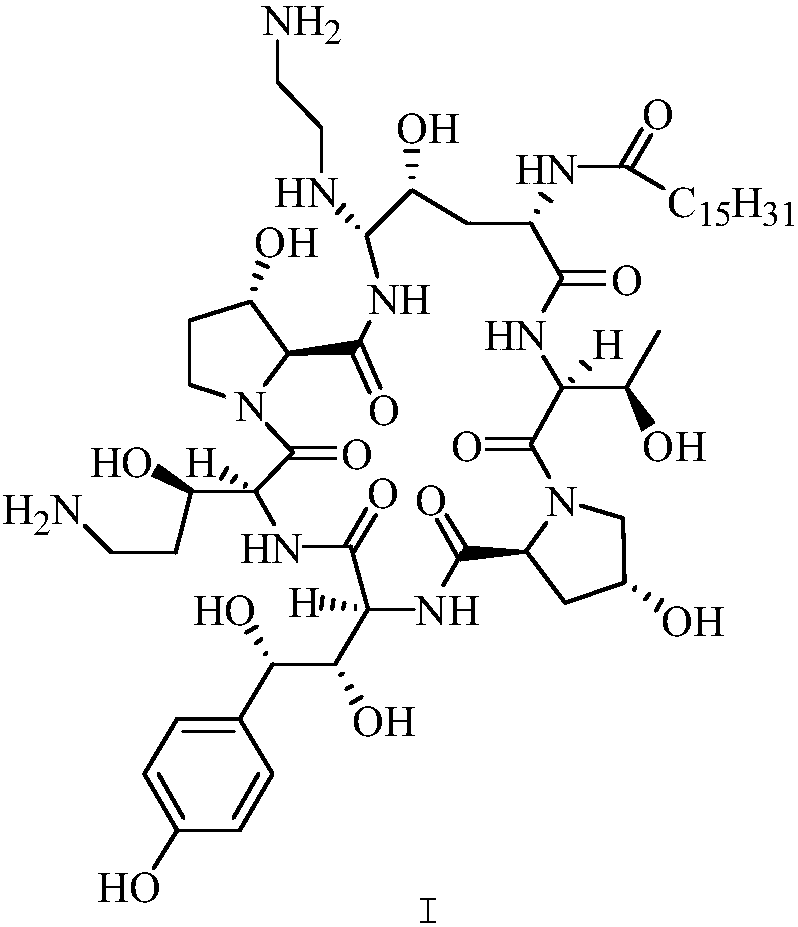
[0032] Under the protection of nitrogen, the compound ...
Embodiment 2
[0034] The compound of formula II (10.00g, 9.4mmol) and dichloromethane (200mL) were placed in a 500mL three-necked flask, under the protection of nitrogen, placed in a low-temperature bath, down to -5°C, and dichloromethane was slowly added dropwise at this temperature Sulfone (SOCl 2 , 0.37g, 3.1mmol) in dichloromethane (20mL) solution, after the dropwise addition, the reaction was started, and the reaction process was tracked and monitored by HPLC, and the reaction time was 3h. Add cold saturated potassium carbonate aqueous solution to the reaction solution until no more bubbles are generated, separate the organic layer, extract the aqueous layer with dichloromethane (50mL×2), combine the organic layers, wash with cold pure water until nearly neutral, and wash with dry Dry over sodium sulfate with water, filter, distill under reduced pressure and dry under vacuum to obtain the compound of formula III as a pale yellow solid. Yield 90.2%
[0035] Under the protection of nit...
Embodiment 3
[0037] The compound of formula II (10.00g, 9.4mmol) and chloroform (200mL) were placed in a 500mL three-neck flask, under the protection of nitrogen, placed in a low-temperature bath, down to -25°C, and slowly added dropwise at this temperature Thionyl chloride ( SOCl 2 , 0.37g, 3.1mmol) in chloroform (20mL) solution, start to react after dropwise addition, HPLC tracking and monitoring reaction process, reaction time 1.5h. Add cold saturated potassium carbonate aqueous solution to the reaction solution until no more bubbles are generated, separate the organic layer, extract the aqueous layer with chloroform (50mL×2), combine the organic layers, wash with cold pure water until nearly neutral, and rinse with anhydrous sulfuric acid Sodium drying overnight, filtration, distillation under reduced pressure and vacuum drying gave the compound of formula III as a pale yellow solid. Yield 90.1%.
[0038] Under the protection of nitrogen, the compound of formula III was dissolved in ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap