Compositions and uses thereof
A technology of compounds and polymerases, which can be used in drug combinations, medical preparations containing active ingredients, tripeptide components, etc., can solve problems such as low specific activity and hindering xenograft human cancer in vivo testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
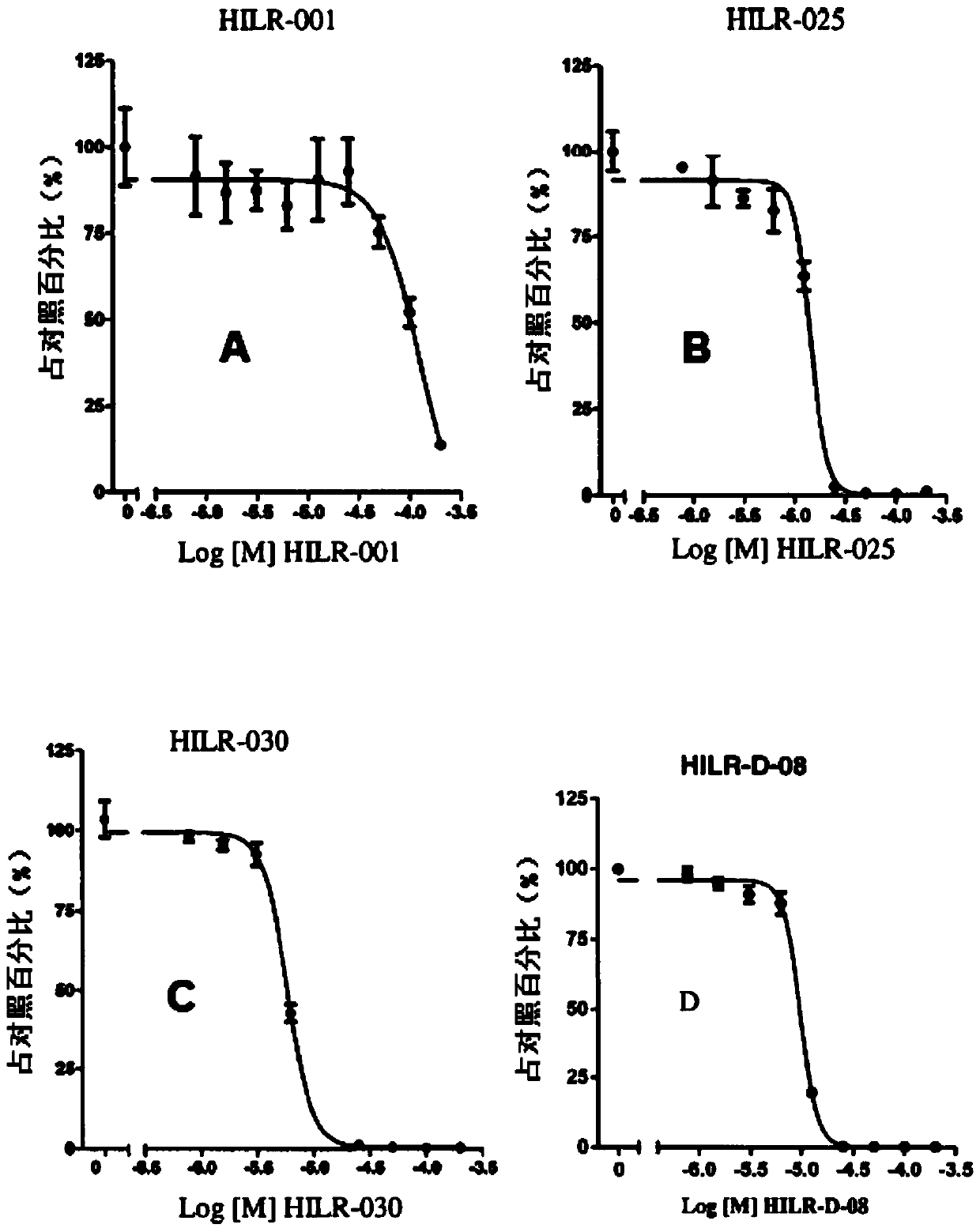
[0372] Example 1: Improved specific activity
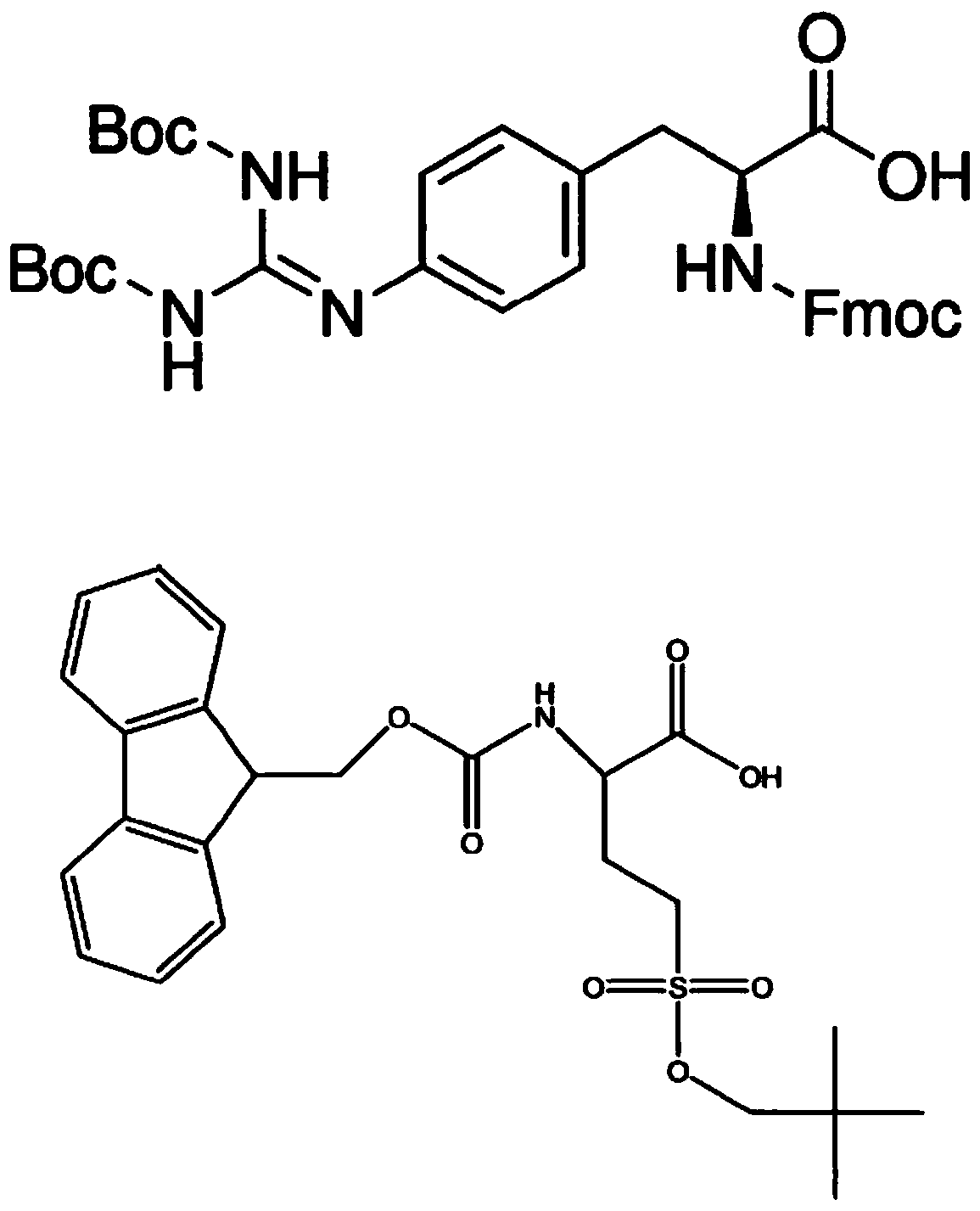
[0373]Three cyclic peptides (HILR-001 (SEQ ID NO: 13), HILR-025 (SEQ ID NO: 15) and HILR-030 (SEQ ID NO: 16)) were prepared with >95% purity using conventional automated peptide synthesis techniques . HILR-001 (SEQ ID NO: 13) is a comparative compound prepared according to the literature [Warenius et al, Molecular Cancer (2011); 10:72-88.]. HILR-025 (SEQ ID NO: 15) and HILR-030 (SEQ ID NO: 16) are cyclic compounds comprising (Trp-Trp-Arg-Arg) or (Trp-Trp-Gpa-Gpa) repeat sequences. The activity of the compounds was tested as follows:
[0374] 1) NCI-H460 cells were grown in Ham's F12 medium supplemented with 10% FBS.
[0375] 2) Cells were harvested and seeded into 96-well plates at 500 cells / well.
[0376] 3) Compounds were prepared from stock solutions and added directly to cells at two-fold dilutions starting at 200 μM. The final DMSO concentration was 0.2%.
[0377] 4) Keep cells and compounds at 37°C, 5% CO 2 , grown ...
Embodiment 2
[0384] Example 2: PARP-dependent cytotoxicity
[0385] The present inventors hypothesized that modulation of PARP activity by the PRGPRP cyclic peptide may at least partially contribute to ATP decline and subsequent necrosis in human NSCLC. Thus the HILRa cyclic peptide may be PARP-dependent. If yes, it is assumed that it should be reversed by a PARP inhibitor such as Olaparib.
[0386] In this case, Olaparib will reduce / prevent cell death induced by HILRa cyclic peptide.
[0387] A study was therefore performed to examine the presence of HILR-001 [cyc-(Pro-Arg-Gly-Pro-Arg-Pro-Val-Ala-Lue-Lys-Leu-Ala-Leu-Lys-Leu-Ala-Leu] (SEQ ID NO: 13) (PolypeptideLaboratories, France, SAS, 7 Rue de Boulogne, 67100, Strasbourg, France)] (alone or co-cultivated with Olaparib) exposed for 72 hours and 96 hours, on NCI-H460 human non-small cell Effects of ATP levels and cell death in lung cancer cells.
[0388] The in vitro PARP standard curve was initially generated [ Figure 5 ].
[03...
Embodiment 3
[0426] Example 3: Competitive inhibition of DEVD
[0427] PARP activity is controlled by the presence or absence of cleavage at the DEVD site. Cleaved PARP is inactive with respect to its poly(ADP-ribose) phosphorylation activity. Poly(ADP-ribose) phosphorylation inhibitors such as olaparib are not expected to have any effect on cleaved PARP. Therefore, it is possible that PRGPRP (SEQ ID NO: 2) acts on intact PARP with an intact DEVD region. Furthermore, it was deduced that the activity of HILR-001 could be explained by PRGPRP (SEQ ID NO: 2) binding to the DEVD region of PARP and thus protecting this region from caspase binding and proteolytic cleavage.
[0428] Irrespective of the general secondary and tertiary conformational orientations of peptide regions, it is noteworthy that the linear alignment of the aspartate anion in the GDEVDG region of PARP (SEQ ID NO: 1) aligns with the cationic arginine very close[ Figure 13 ], and these arginines have been shown to be criti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com