Processes to prepare elongated 2-ketoacids and C5-C10 compounds therefrom via genetic modifications to microbial metabolic pathways
A C6-C10, gene modification technology, applied in the field of microorganisms of isopropylmalate synthase, can solve the problem that microorganisms cannot produce
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
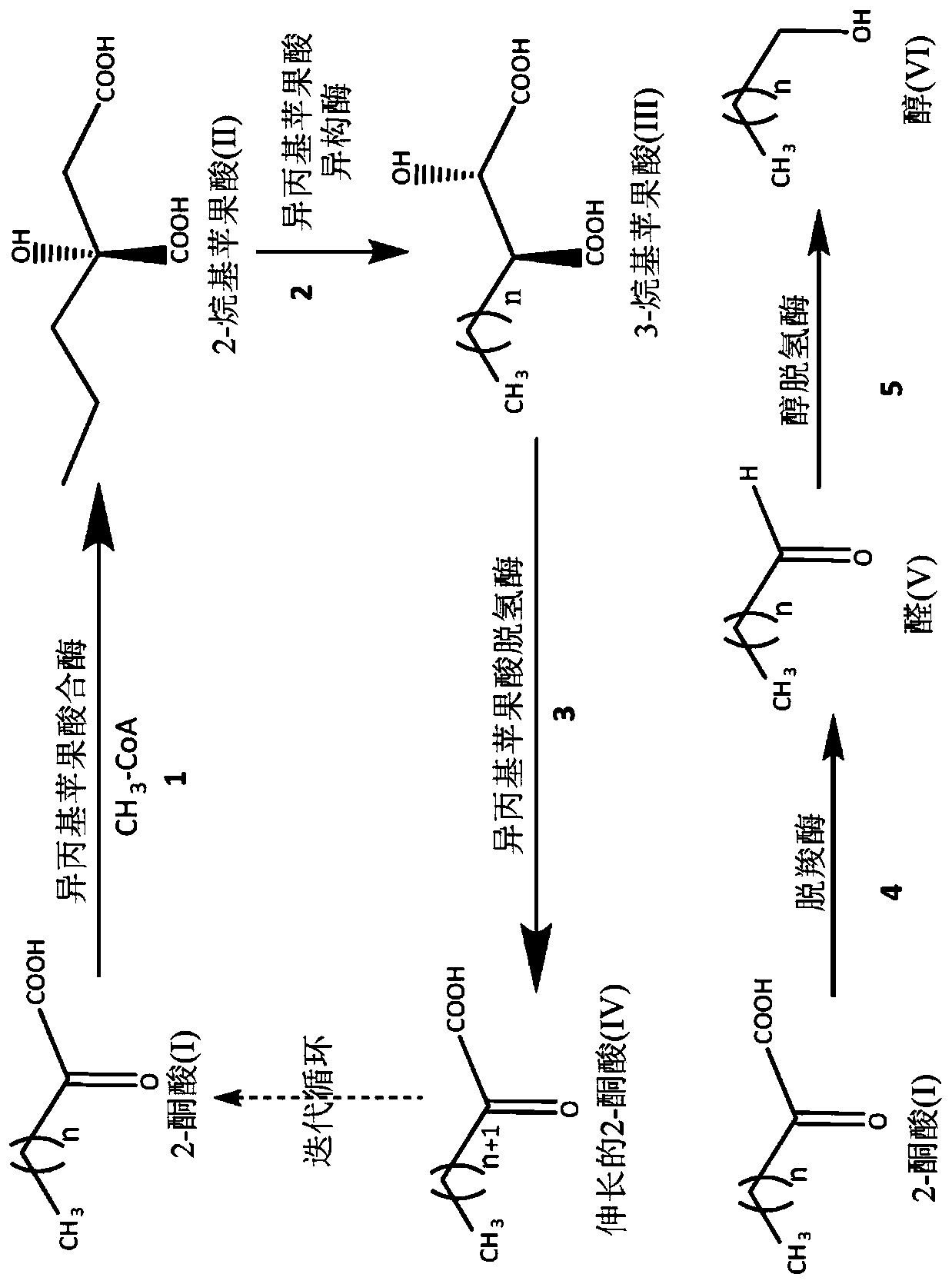
[0070] Preparation of a genetically modified isopropylmalate synthase with enhanced catalytic activity towards 2-ketohexanoate and 2-ketooctanoate.
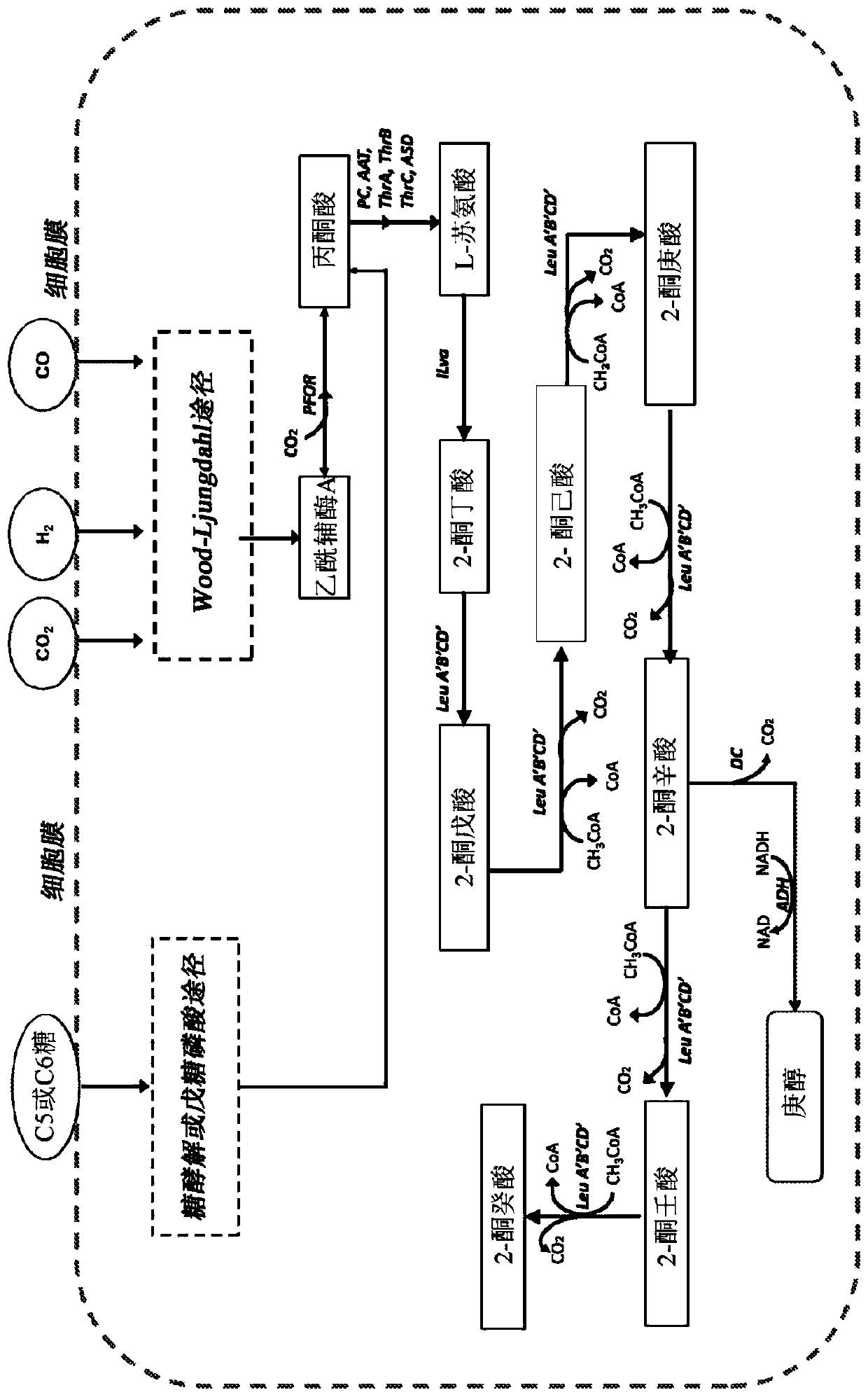
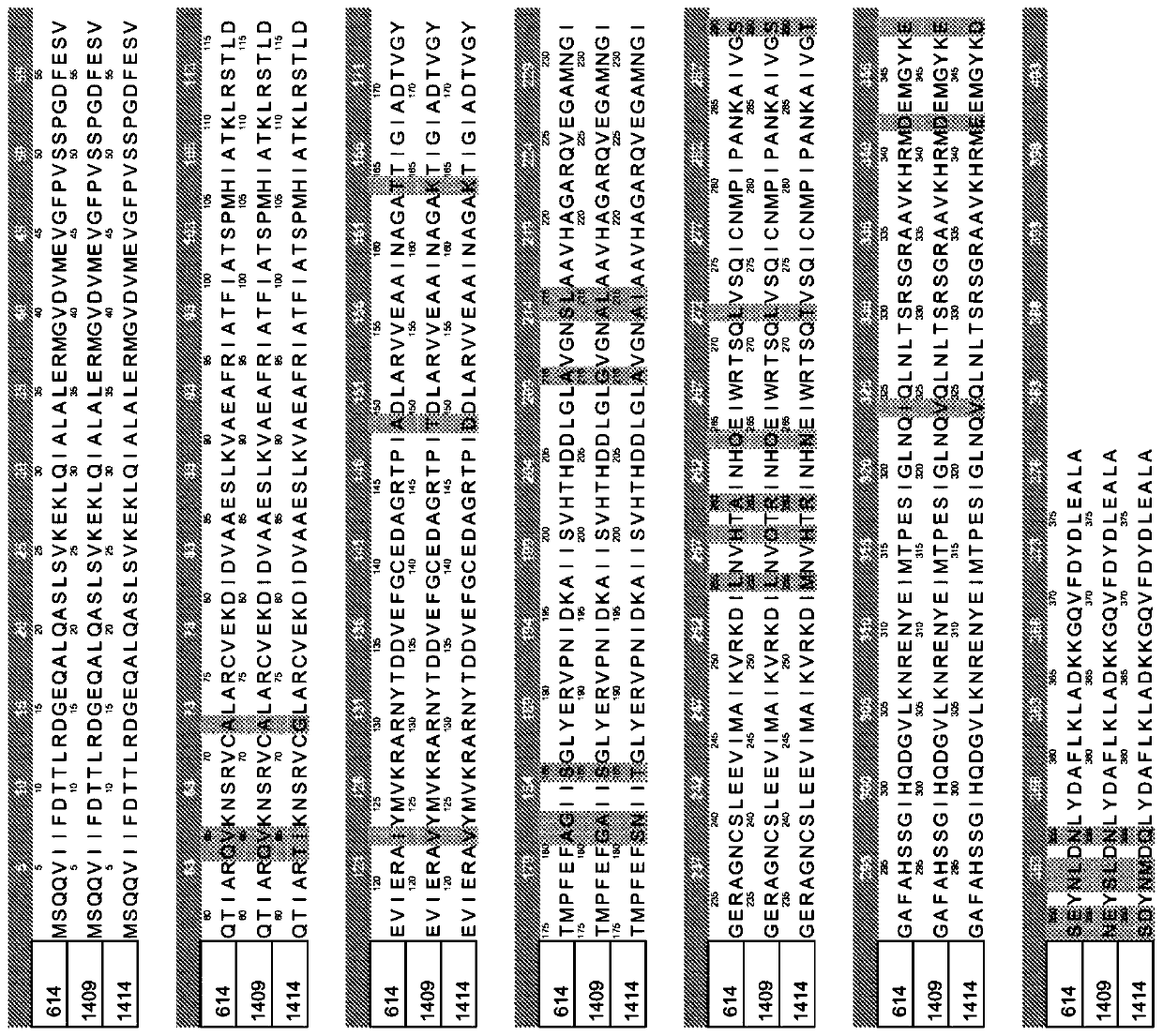
[0071] During the biosynthesis of 2-ketononanoic acid via the recursive activity of the "+1" pathway of the leucine biosynthetic pathway, isopropylmalate synthase traps and condenses acetyl-CoA and 2-ketoacids of various lengths. For efficient biosynthesis of 2-ketononanoic acid, isopropylmalate synthase is expected to efficiently capture 2-ketobutyrate, 2-ketovalerate, 2-ketohexanoate, 2-ketoheptanoate and 2-ketoheptanoate with acetyl-CoA. and / or 2-ketooctanoic acid, resulting in the corresponding 2-alkylmalic acid product ( figure 1 Intermediate II) in. The native isopropylmalate synthase of E. coli and other microorganisms is less efficient at capturing longer unnatural 2-ketoacid substrates. To increase the activity of native isopropylmalate synthase to capture longer 2-keto acids for catalysis, the active site of isopropy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com