Lithium recycling method in lithium-containing waste
A technology of waste and content, applied in recycling technology, recycling of waste collectors, battery recycling, etc., can solve problems such as hardening, achieve the effects of lowering reaction temperature, good thermal conductivity, and eliminating hardening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The recycling method of lithium in this lithium-containing waste material comprises the following steps:
[0024] A. Mix the lithium-containing waste material with graphite, so that the lithium-containing waste material is doped with a certain proportion of graphite;
[0025] B. the mixed waste obtained in step A is reacted with an acidic substance;
[0026] C. heat-treating the product obtained in step B to generate insoluble oxides and soluble lithium salts.
[0027] D. Leaching and solid-liquid separation of the product obtained in step C through an aqueous solution to obtain a lithium-rich solution and residue.
[0028] F. Add soluble salt or / and soluble base to the lithium-enriched solution obtained in step D, and filter to obtain lithium salt precipitation or directly obtain lithium salt crystals by evaporation or freezing. Acidic substances undergo a displacement reaction with mixed waste
[0029] LiCoO2+H+=HCoO2+Li+
[0030] Li2CO3+2H+=H2CO3+2Li+
[0031] I...
Embodiment 2
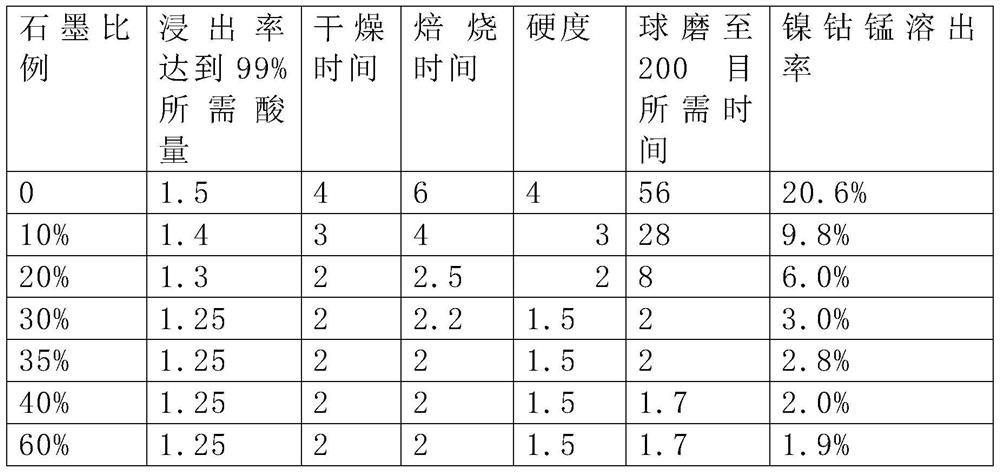
[0044] The only difference between this example and Example 1 is that the graphite content reaches 20%, and concentrated sulfuric acid is added according to the molar ratio of H / Li=1.3 (when H / Li=1.2, the lithium leaching rate is only 96%), and the reaction system is mixed. The temperature rises sharply, up to 117°C, and the material agglomerates. In order to make the reaction system uniform, it needs to be ball milled to 200 mesh, and it takes at least 8 hours to grind the material to 200 mesh.
[0045] Then carry out roasting treatment, the roasting temperature is set to 500 degrees, it needs roasting for at least 2.4 hours to make the lithium leaching rate above 99%, after the roasting is completed, the material has agglomerated, the measured hardness is 2, and the time required for ball milling to 200 mesh for 8h. Leaching with water, by detecting the transition metal content of the leaching solution, 4.2% of the transition metal was dissolved.
Embodiment 3
[0047]The only difference between this example and Example 1 is that the graphite content reaches 30%, and concentrated sulfuric acid is added according to the molar ratio of H / Li=1.1 (when H / Li=1.08, the lithium leaching rate is only 96%), and the reaction system is mixed. The temperature rises sharply, up to 90°C, and the material agglomerates. In order to make the reaction system uniform, it needs to be ball milled to 200 mesh, and it takes at least 2 hours to grind the material to 200 mesh.
[0048] Then carry out roasting treatment, the roasting temperature is set to 500 degrees, it needs roasting for at least 2.2 hours to make the leaching rate of lithium more than 99%, after the roasting is completed, the material has agglomerated, the measured hardness is 1.5, and the time required for ball milling to 200 mesh for 2h. Leaching with water, by detecting the transition metal content of the leach solution, 1.2% of the transition metal was dissolved.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com