Therapeutic agent for amyotrophic lateral sclerosis and composition for treatment
A technology for lateral sclerosis and therapeutic agent, applied in the field of amyotrophic lateral sclerosis therapeutic agent and ALS therapeutic composition, can solve the problem that there is no ALS model, SOD1-ALS model cannot function as a model, and the drug Issues not yet developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0072] In one embodiment, the present invention provides a method for treating ALS, which includes the following steps: administering an effective amount of the compound represented by the above formula (1), its pharmaceutically acceptable salt, or their of solvates. In the treatment method of this embodiment, examples of the compound represented by the above formula (1), its pharmaceutically acceptable salt, or their solvate include the same ones as those described above.
[0073] In one embodiment, the present invention provides a compound represented by the above formula (1), a pharmaceutically acceptable salt thereof, or a solvate thereof for use in the treatment of ALS. In the treatment method of this embodiment, examples of the compound represented by the above formula (1), its pharmaceutically acceptable salt, or their solvate include the same ones as those described above.
[0074] In one embodiment, the present invention provides the use of the compound represented b...
experiment example I-1
[0078] (differentiate into motor neurons)
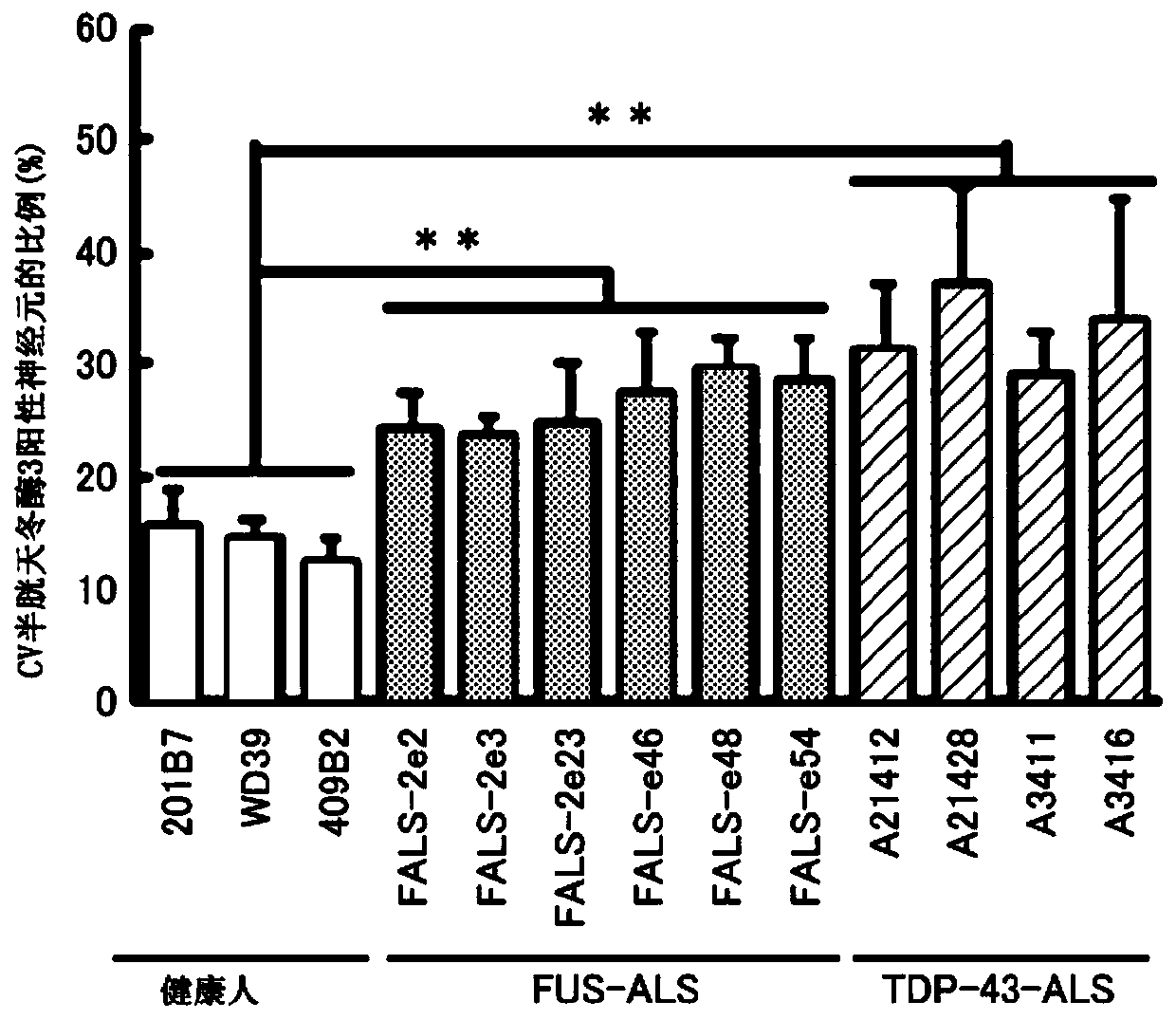
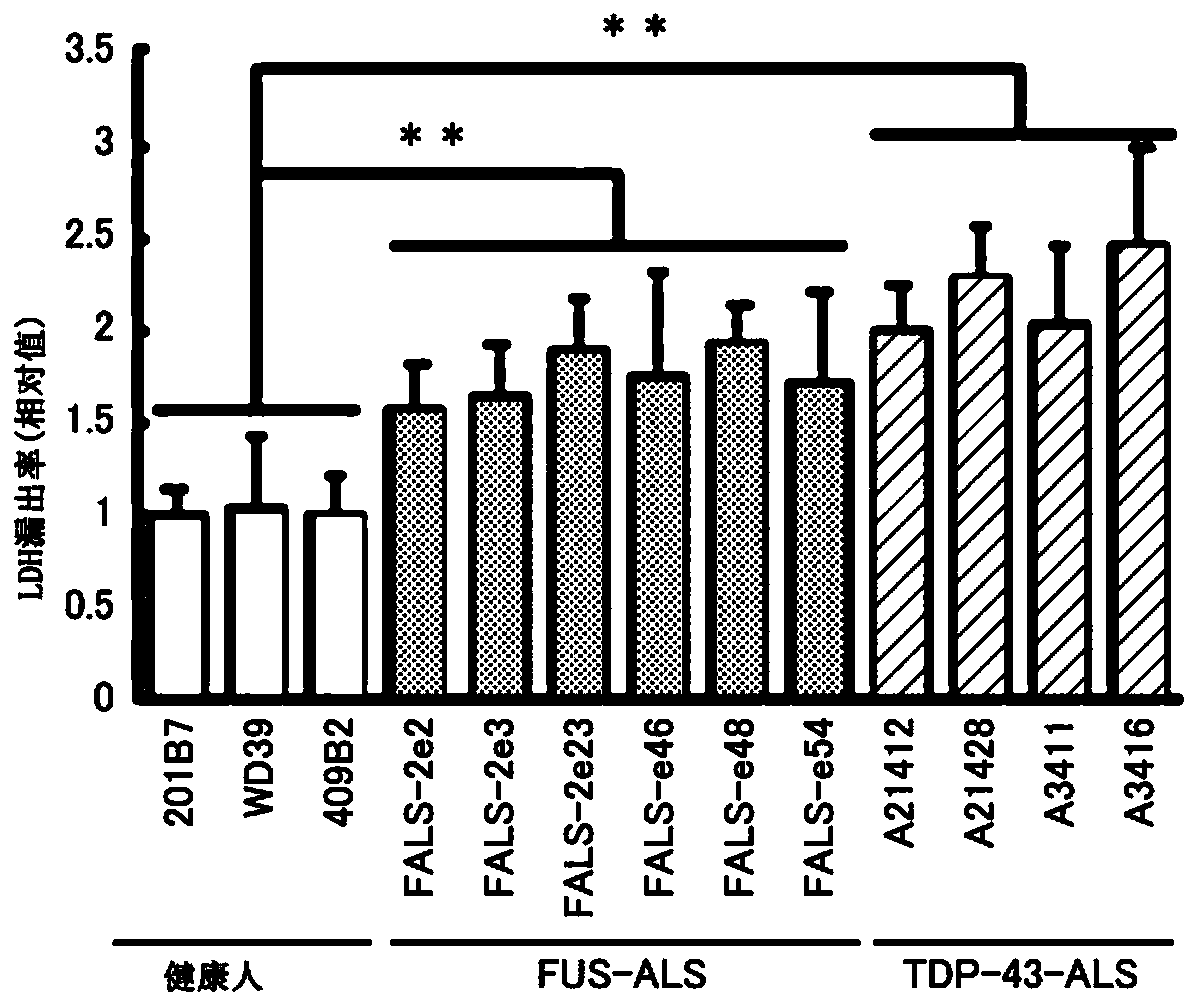
[0079] Among familial ALS, ALS due to mutation of FUS gene and ALS due to mutation of TDP-43 gene are known to exist.
[0080] Then, iPS cells derived from a healthy person, iPS cells derived from an ALS patient with a FUS mutation, and iPS cells derived from an ALS patient with a TDP-43 mutation were differentiated into motor neurons. The iPS cell lines used are as described in Table 1, all of which are derived from skin fibroblasts.
[0081] [Table 1]
[0082]
[0083] Specifically, first, each of the above cell lines was mixed with SB431542 (CAS number: 301836-41-9) at a final concentration of 3 μM, CHIR99021 (CAS number: 252917-06-9) at a final concentration of 3 μM, and Dorsomorphin (CAS number: 866405-64-3) at a concentration of 3 μM was cultured for 5 days to induce differentiation-promoted pluripotent stem cells (DiSC). Medium was exchanged daily. Hereinafter, the start of differentiation induction refers to the start ...
experiment example I-2
[0099] (Analysis of neurite length)
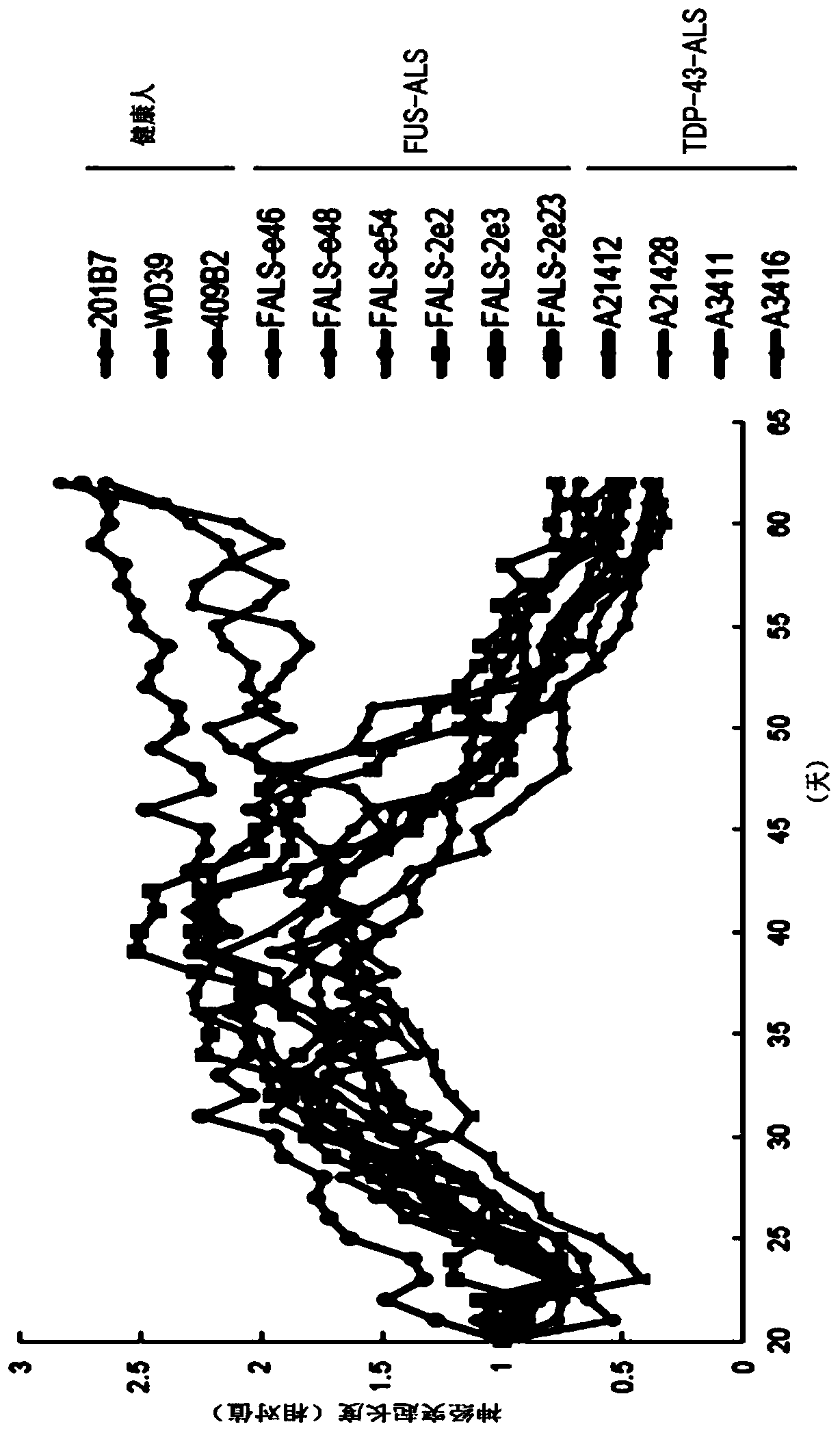
[0100] For each motor neuron induced to differentiate in Experimental Example I-1, the temporal change in neurite length was measured. Biostation CT (Nikon) was used for the temporal measurement of neurite length. figure 1 is a graph showing the measurement results of neurite length changes over time. The vertical axis shows neurite length (relative value), and the horizontal axis shows the number of days of culture from the initiation of differentiation induction. As a result, it was clarified that in motor neurons induced by differentiation of iPS cells derived from healthy individuals, neurite length continued to extend, whereas in motor neurons induced by differentiation of iPS cells derived from ALS patients, The neurite length which is the peak at about day 40 from the initiation of differentiation induction shortens. The results show that differentiation-induced motor neurons reflect the pathology of ALS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com