Combination drug for treating leukemia and application of combination drug in preparation of drug for treating acute myeloid leukemia
An acute myeloid and leukemia technology, applied in the field of medicine, can solve relapse and refractory problems, and achieve the effect of solving relapse and refractory problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Clinical trial of Chidamide combined with DCAG in the treatment of acute myeloid leukemia
[0038] 1.1 Test drugs
[0039] Cidabenamide tablets (produced by Shenzhen Microchip Biotechnology Co., Ltd.), decitabine for injection (produced by Lunan Pharmaceutical Group Co., Ltd.), aclamycin for injection, cytarabine for injection, and Recombinant human granulocyte stimulating factor injection (purchased from hospital pharmacy, no fixed supplier).
[0040] 1.2 Test method
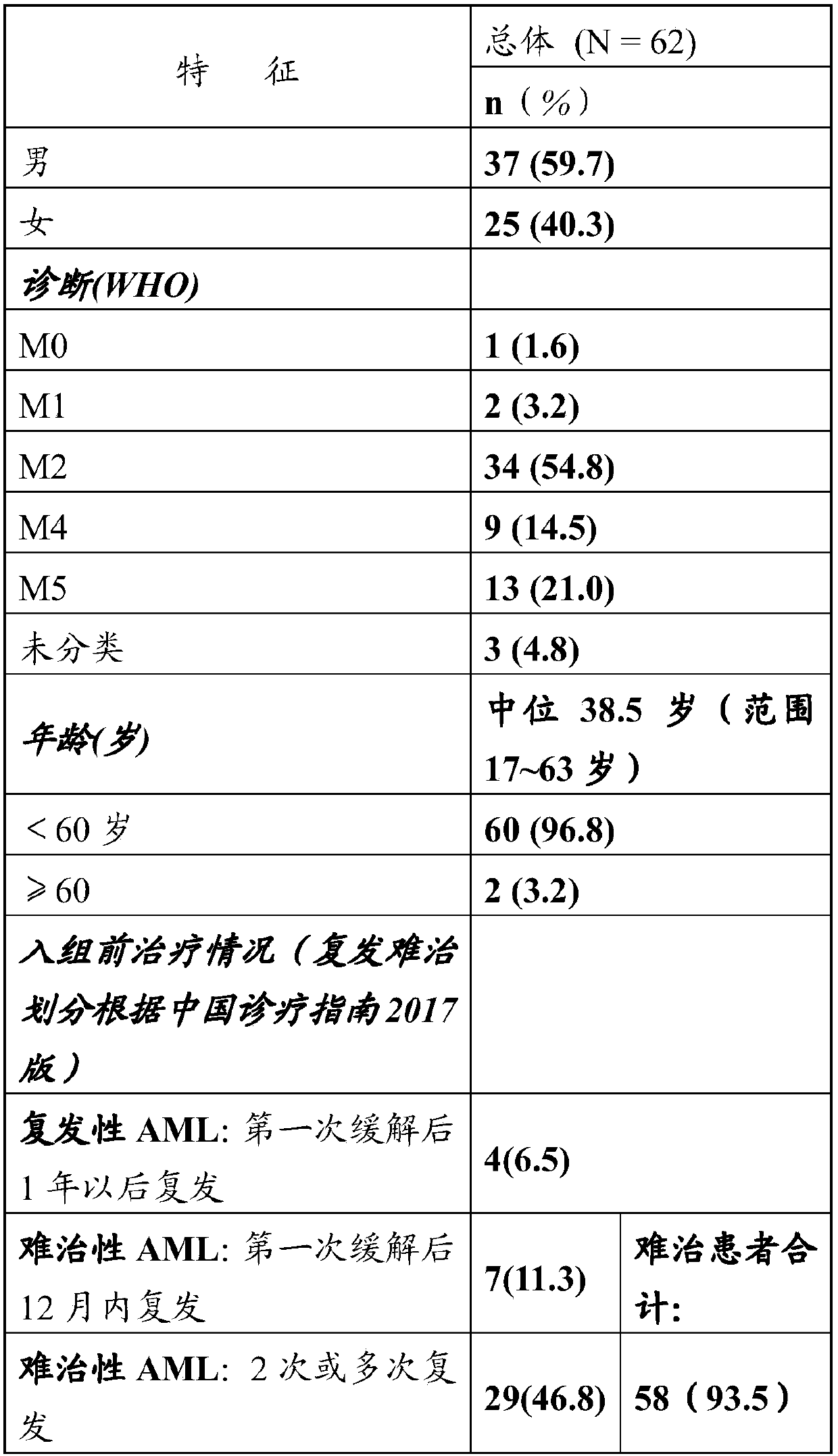
[0041] Number of cases: 100 cases in total;
[0042] standard constrain:
[0043] 1) Between the ages of 18-59, there is no limit to men or women;
[0044] 2) AML patients (non-AML-M3) diagnosed according to the 2008 World Health Organization (WHO) diagnostic criteria for myeloid malignancies;
[0045] 3) Patients who have undergone at least one systemic treatment (including chemotherapy, hematopoietic stem cell transplantation, etc.) without remission or relapse after remission;
[0046] 4) The ECOG behav...
Embodiment 2
[0115] Example 2: Clinical trial of Chidamide combined with DCAG in the treatment of acute myeloid leukemia
[0116] 2.1 Test drugs, 2.2 Test methods are the same as those in Example 1.
[0117] 2.3 The clinical trial results of chidamide combined with DCAG in the treatment of acute myeloid leukemia are as follows:
[0118] This clinical trial has increased the number of evaluable cases to 93 on the basis of Example 1. The results are as follows:
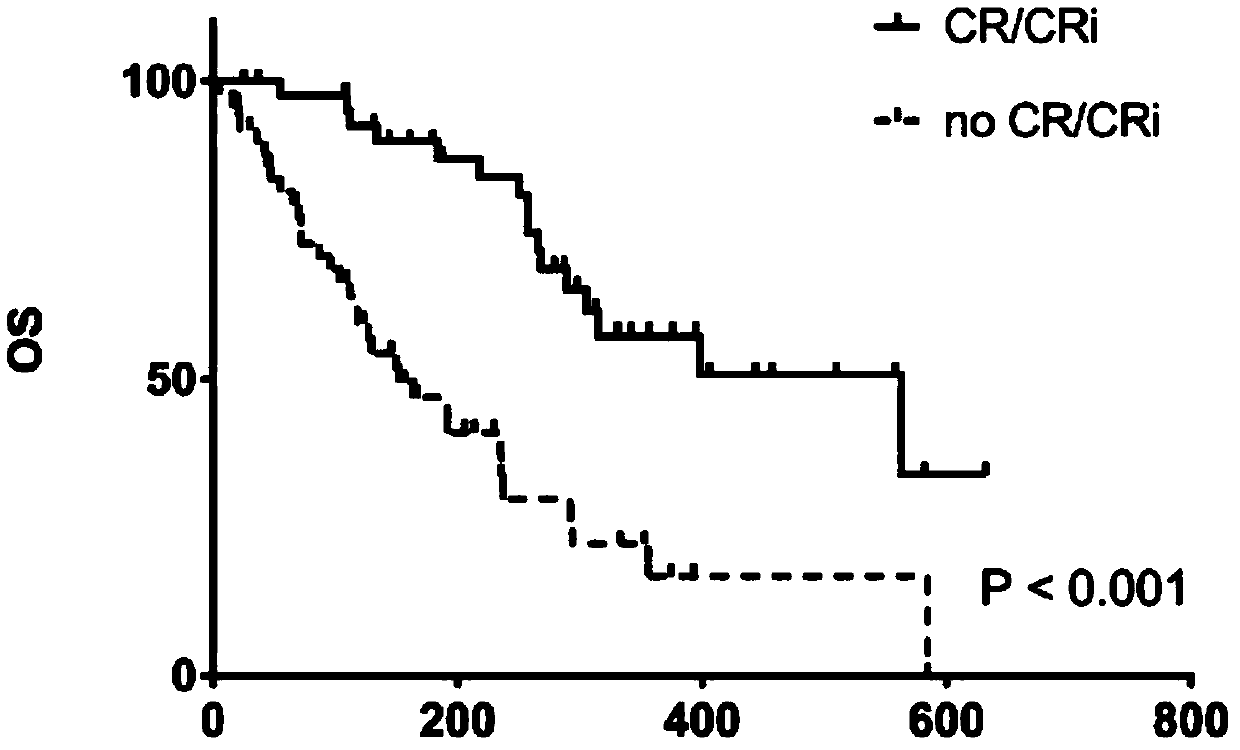
[0119] Clinical trial results: the overall number of evaluable persons (n=93), of which 24 cases achieved complete remission, the CR rate was 26% (24 / 93, 95% confidence interval [CI]: 16.9–34.7%), and 19 cases achieved complete remission. Remission with incomplete recovery of peripheral blood (CRi for short), the CRi rate was 20% (19 / 93, 95% CI: 12.2-28.4%), and the objective total effective rate was 46% (95% CI: 36.1-56.4%), The clinical trial results are shown in Table 5.
[0120] Table 5: Clinical trial results *
[0121]
[0122] * The t...
Embodiment 3
[0125] Example 3: Combined pharmaceutical composition
[0126] The combined pharmaceutical composition of this embodiment contains the following effective doses of active ingredients:
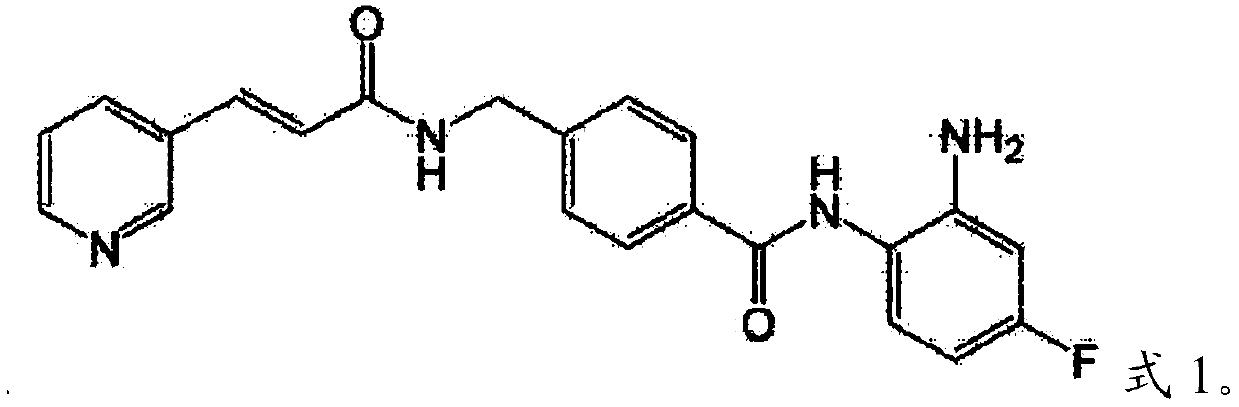
[0127] Chidamide 180mg, Decitabine 20mg / m 2 , Aclarithromycin 10mg / m 2 , Cytarabine 100mg / m 2 And granulocyte colony stimulating factor 300ug / day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com