A novel thiazole drug molecule for hospital disinfection and its preparation method
A technology of drug molecules and thiazoles, which is applied in botany equipment and methods, chemicals for biological control, disinfectants, etc., can solve the problems that affect the appearance of medical devices, low molecular polarity, low solubility, etc., and achieve Good force, good inhibition, safe reaction and operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
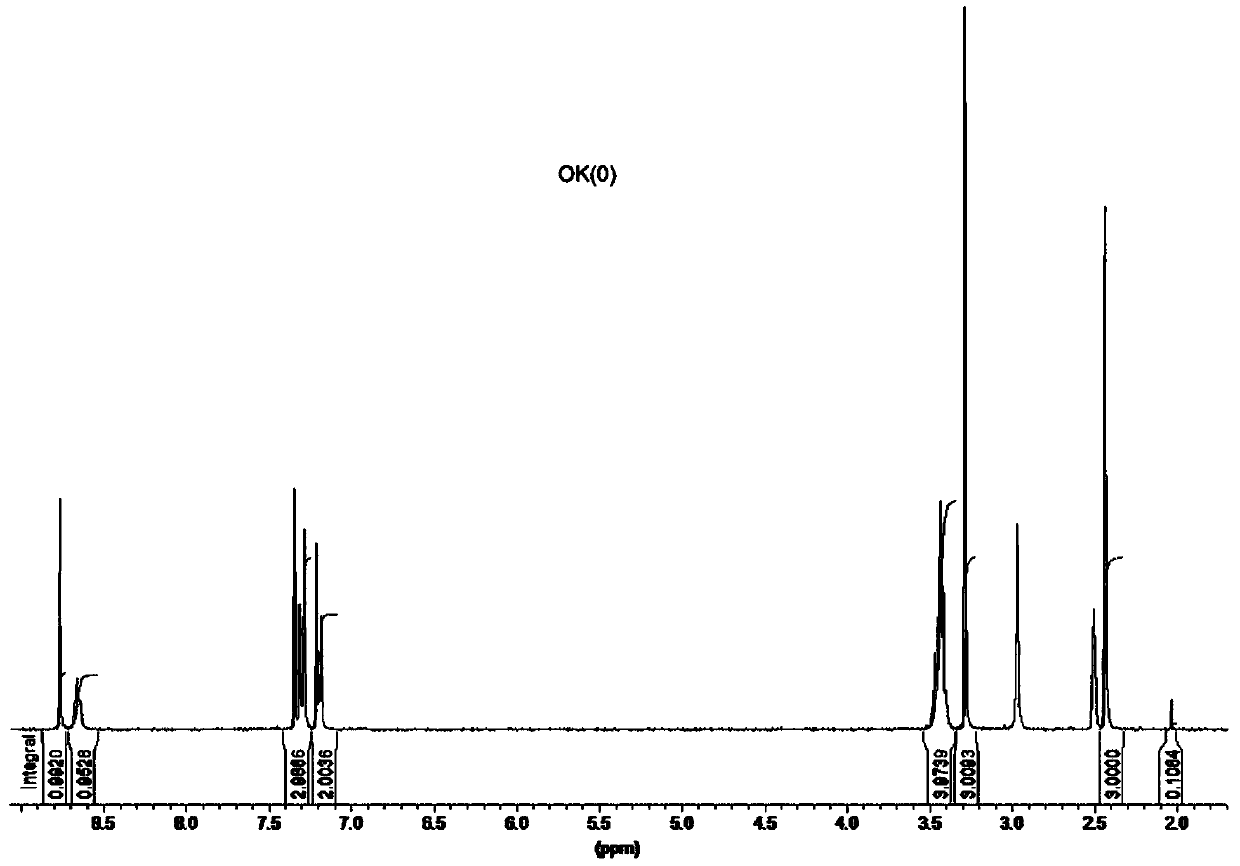
[0030]In a reaction device with a water separator, add 13.5g of 4-methylacetophenone, 11.5g of thiourea and 38g of elemental iodine into 200mL of N,N-dimethylformamide, slowly raise the temperature to 100°C, and react for 2h Finally, 120 mL of the reaction solvent was aliquoted under vacuum conditions, the reaction system was in a viscous state, cooled to room temperature, 150 mL of ether was added to the reaction system, a large amount of solids were precipitated, the reaction solution was filtered, all the filter cake was added to 300 mL of water, and heated to reflux , reacted for 30 minutes, cooled to 10 ° C, a large amount of solids precipitated, filtered the reaction again, and dried the filter cake to obtain 17 g of compound 4-(4-methylbenzene)-thiazol-2-amine; 1 H NMR (400MHz, DMSO-d 6 ):δ7.67(dd,J 1 =8.0Hz,J 2 =4.0Hz, 2H), 7.16(d, J=8.0Hz, 2H), 7.01(s, 2H), 6.90(s, 1H), 2.35(s, 3H); 13 C NMR (400MHz, DMSO-d 6 ): 168.2, 149.9, 136.4, 132.3, 129.0, 125.5...
Embodiment 2
[0032] In a reaction device with a water separator, add 13.5g of 4-methylacetophenone, 11.5g of thiourea and 25g of elemental iodine into 170mL of N,N-dimethylformamide, slowly raise the temperature to 100°C, and react for 2h Finally, 120 mL of the reaction solvent was aliquoted under vacuum conditions, the reaction system was in a viscous state, cooled to room temperature, 150 mL of ether was added to the reaction system, a large amount of solids were precipitated, the reaction solution was filtered, all the filter cake was added to 300 mL of water, and heated to reflux , reacted for 30 minutes, cooled to 10 ° C, a large amount of solids precipitated, filtered the reaction again, and dried the filter cake to obtain 14.3 g of compound 4-(4-methylbenzene)-thiazol-2-amine; 1 H NMR (400MHz, DMSO-d 6 ):δ7.67(dd,J 1 =8.0Hz,J 2 =4.0Hz, 2H), 7.16(d, J=8.0Hz, 2H), 7.01(s, 2H), 6.90(s, 1H), 2.35(s, 3H); 13 C NMR (400MHz, DMSO-d 6 ): 168.2, 149.9, 136.4, 132.3, 129.0, 125.5, 100.6, ...
Embodiment 3
[0034] In a reaction device with a water separator, add 13.5g of 4-methylacetophenone, 7.6 thiourea and 38g of elemental iodine into 200mL of N,N-dimethylformamide, slowly raise the temperature to 100°C, and react for 2h Finally, 120 mL of the reaction solvent was aliquoted under vacuum conditions, the reaction system was in a viscous state, cooled to room temperature, 150 mL of ether was added to the reaction system, a large amount of solids were precipitated, the reaction solution was filtered, all the filter cake was added to 300 mL of water, and heated to reflux , reacted for 30 minutes, cooled to 10°C, a large amount of solids were precipitated, the reaction was filtered again, and the filter cake was dried and separated by silica gel column chromatography to obtain 9.2 g of compound 4-(4-methylbenzene)-thiazol-2-amine; 1 H NMR (400MHz, DMSO-d 6 ):δ7.67(dd,J 1 =8.0Hz,J 2 =4.0Hz, 2H), 7.16(d, J=8.0Hz, 2H), 7.01(s, 2H), 6.90(s, 1H), 2.35(s, 3H); 13 C NMR (400MHz, DMSO-d ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com