High-air-stability inorganic sulfide solid electrolyte and preparation method and application thereof
A sulfide electrolyte, inorganic sulfide technology, applied in solid electrolyte, electrolyte battery manufacturing, inorganic chemistry and other directions, can solve the problems of restricting the application of all-solid-state lithium batteries, unstable inorganic sulfide electrolyte, and reduced structural ionic conductivity, etc. Achieve the effects of excellent ionic conductivity, good air stability and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
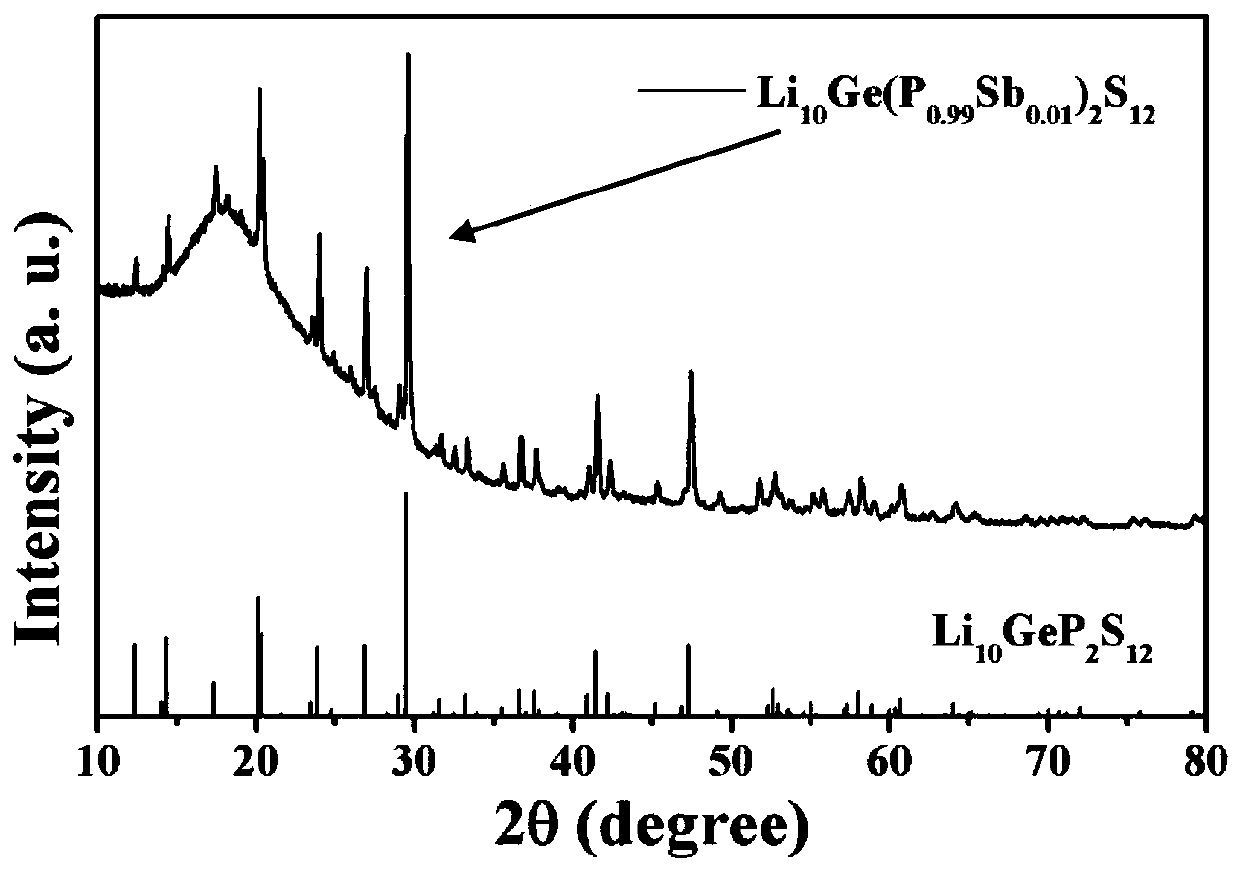
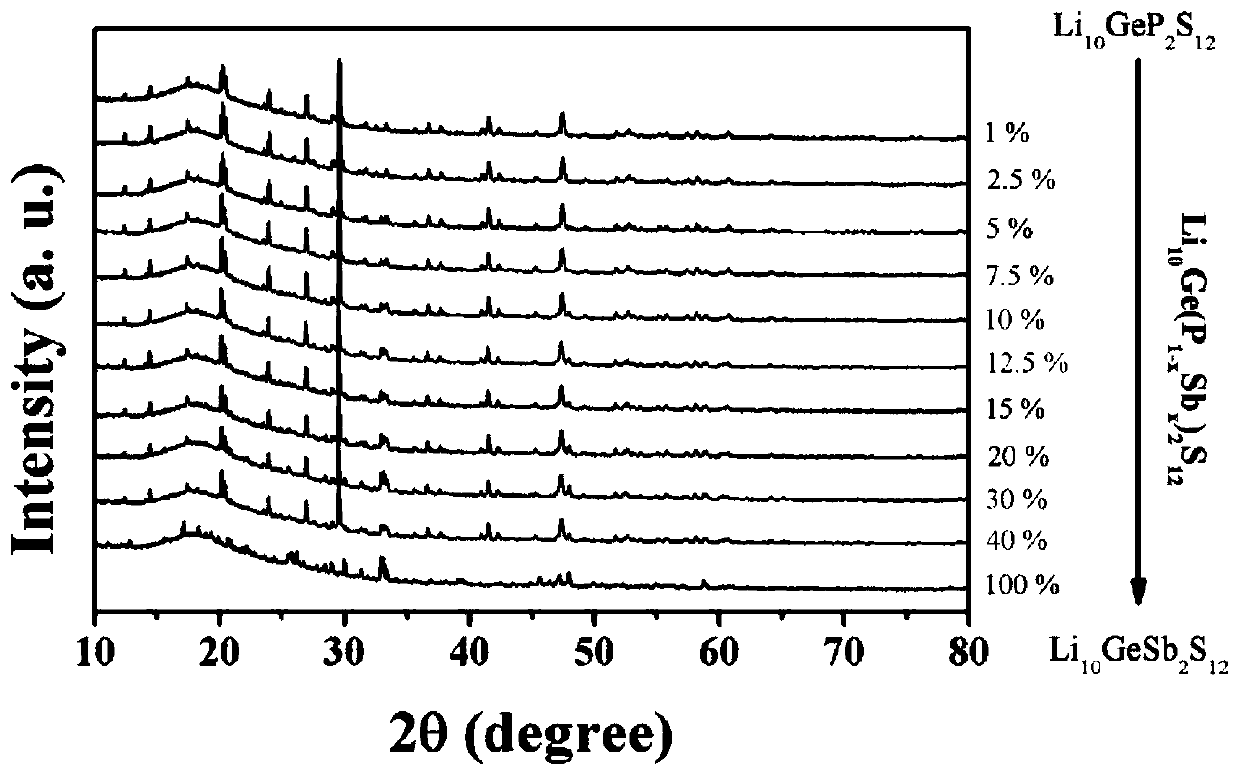
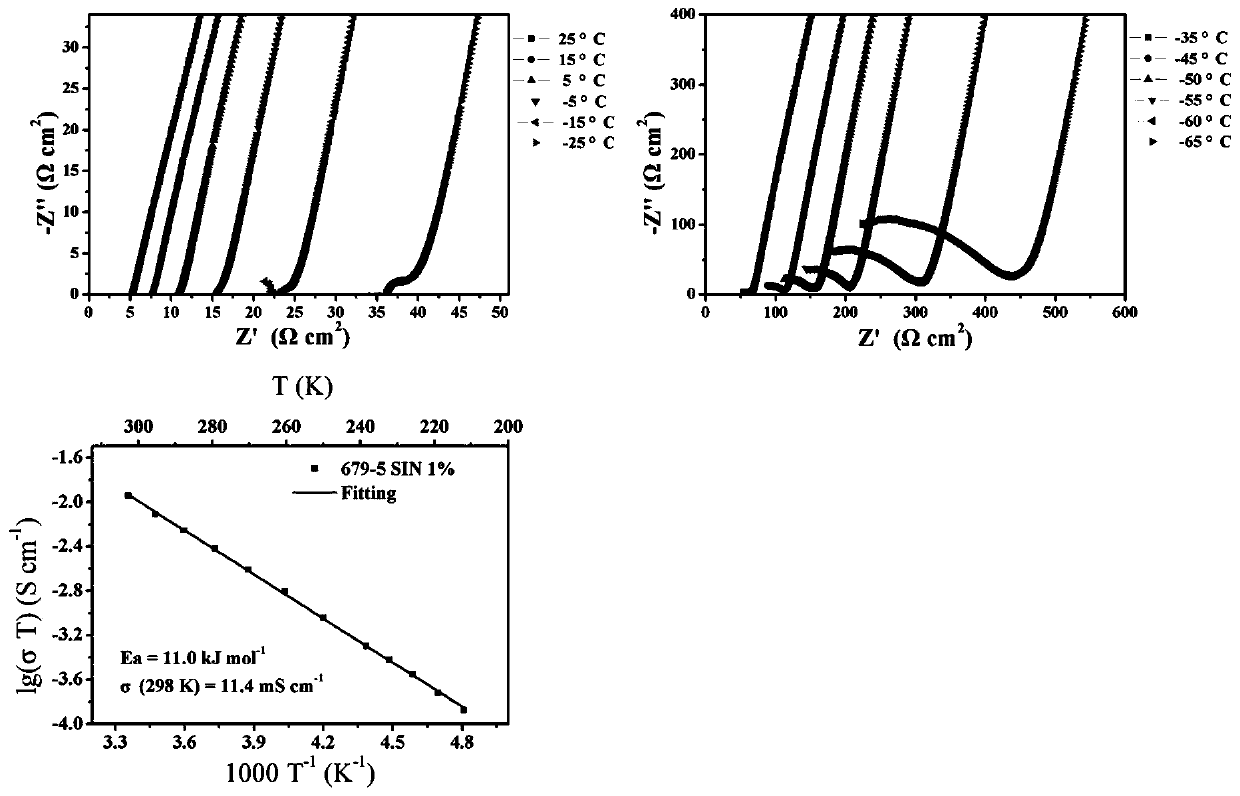
[0053] Embodiment 1 prepares Li 10 Ge(P 1-a Sb a ) 2 S 12 Solid electrolyte material (0.01≤a≤1)
[0054] 15 mmol Li 2 S (0.69 g), 3 mmol of GeS 2 (0.411 g), (3-3a) mmol of P 2 S 5 , 3a mmoles of Sb 2 S 5 The powder is ground and mixed in a mortar, where 0.01≤a≤1. When a=0.01, the batching of each raw material is as follows: Li 2 S is 0.69 g, GeS 2 is 0.411 g, P 2 S 5 0.66 g, Sb 2 S 5 is 0.012 grams; as a=0.1, the batching of each raw material is as follows: Li 2 S is 0.69 g, GeS 2 is 0.411 g, P 2 S 5 0.599 g, Sb 2 S 5is 0.121 grams. And so on. After grinding and mixing, put it into a 50 milliliter zirconia ball mill jar for ball milling, the ball milling speed is 400 rpm, and the ball milling time is 12 hours. The ball-milled samples were pressed into discs using a powder tablet press at a pressure of 100 MPa, and then sealed in a vacuum quartz tube for calcination. The calcination temperature is controlled by a temperature program, from room temperatu...
Embodiment 2
[0056] Embodiment 2 prepares Li 6 (P 1-a Sb a )S 5 Cl solid electrolyte material (0.01≤a≤1)
[0057] 20 mmol Li 2 S (0.92 g), 8 mmol of LiCl (0.336 g), (4-4a) mmol of P 2 S 5 and 4a mmol of Sb 2 S 5 The powder is ground and mixed in a mortar, where 0.01≤a≤1. When a=0.025, the batching of each raw material is as follows: Li 2 S is 0.92g, LiCl is 0.336g, P 2 S 5 0.866 g, Sb 2 S 5 is 0.03 grams; as a=0.1, the batching of each raw material is as follows: Li 2 S is 0.92g, LiCl is 0.336g, P 2 S 5 0.799 g, Sb 2 S 5 is 0.121 grams. And so on. After grinding and mixing, put it into a 50 milliliter zirconia ball mill jar for ball milling, the ball milling speed is 400 rpm, and the ball milling time is 12 hours. The ball-milled samples were pressed into discs using a powder tablet press at a pressure of 100 MPa, and then sealed in a vacuum quartz tube for calcination. The calcination temperature is controlled by a temperature program, from room temperature to 550 deg...
Embodiment 3
[0059] Embodiment 3 prepares Li 10 Sn(P 0.95 Sb 0.05 ) 2 S 12 solid electrolyte material
[0060] 15 mmol Li 2 S (0.69 g), 3 mmol of SnS 2 (0.549 g), 2.85 mmol of P 2 S 5 (0.633 g), 0.15 mmol of Sb 2 S 5 (0.061 g) powder was ground and mixed in a mortar and pestle. After grinding and mixing, put it into a 50 milliliter zirconia ball mill jar for ball milling, the ball milling speed is 400 rpm, and the ball milling time is 12 hours. The ball-milled samples were pressed into discs using a powder tablet press at a pressure of 100 MPa, and then sealed in a vacuum quartz tube for calcination. The calcination temperature is controlled by a temperature program, from room temperature to 550 degrees Celsius over 4 hours, and kept at this temperature for 4 hours, and then controlled to cool down to 50 degrees Celsius for 4 hours to obtain Li 10 Sn(P 0.95 Sb 0.05 ) 2 S 12 solid electrolyte material.
[0061] Figure 9 for Li 10 Sn(P 0.95 Sb 0.05 ) 2 S 12 X-ray diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com