Monocyclic monoterpene glycoside compound and its preparation method and use
A technology of monoterpene glycosides and compounds, applied in the field of three monocyclic monoterpene glycosides and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
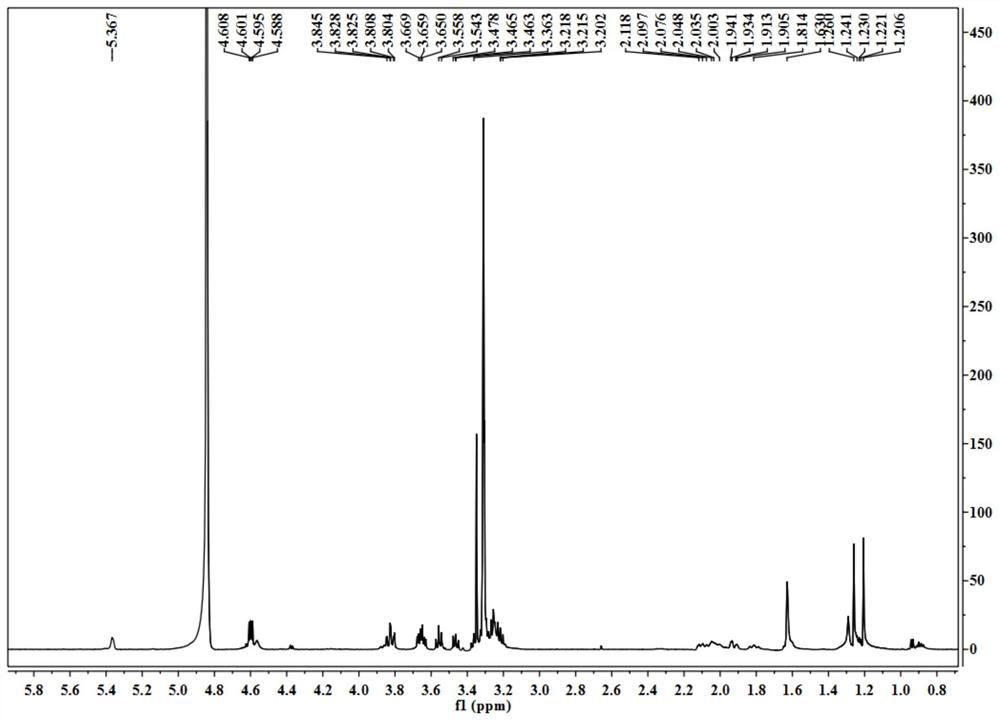
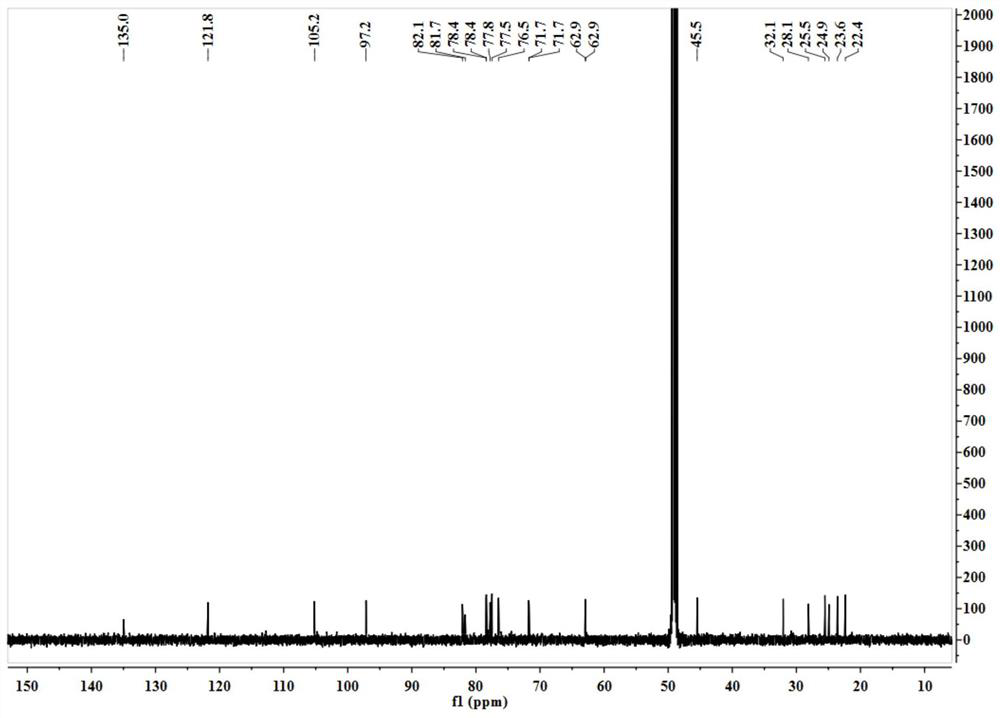
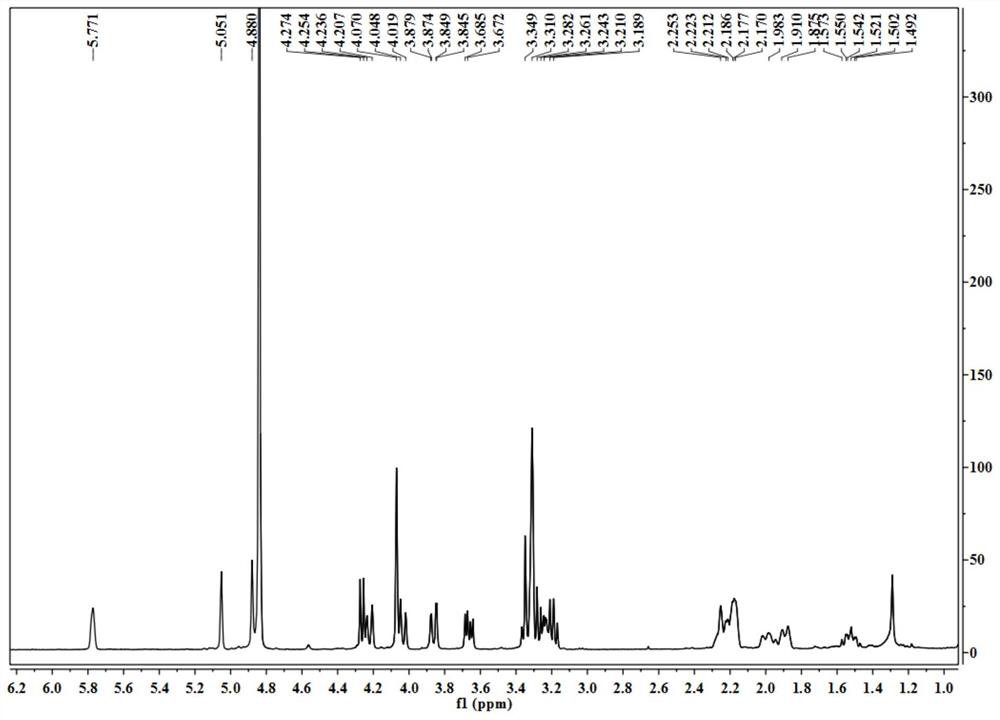
[0037] A, get the aerial part 2kg of blue orchid plant Dracocephalum komarovi Lipsky, after pulverizing with 5 times the volume concentration of 70% ethanol aqueous solution at room temperature, cold immersion extraction is 3 times, and the solvent is evaporated to dryness under reduced pressure to obtain extract;
[0038] b. The extract obtained in step a is separated by a normal-phase silica gel column, and the gradient elution is carried out with n-hexane-ethyl acetate in a volume ratio of 100:1-1:1, and the fraction is analyzed by silica gel thin layer chromatography (TLC) , the same fractions were combined to obtain 9 components (F1-F9); component F4 was subjected to reverse phase silica gel column chromatography, eluted with methanol-water with a volume ratio of 10%-100%, and the obtained fractions were Prepare a reversed-phase column (C 18 , 5μm, 10 × 150mm), separated by isocratic elution with methanol-water solution with a concentration of 40% to obtain the compound o...
Embodiment 2
[0040] A, get the aerial part 2kg of blue orchid plant Dracocephalum komarovi Lipsky, after pulverizing, use 6 times of volume concentration to be 70% ethanol-water solution temperature 80 ℃ of reflux extraction 3 times, evaporate the solvent under reduced pressure to obtain extract;
[0041] b. The extract obtained in step a is separated with a normal-phase silica gel column, and the gradient elution is carried out with cyclohexane-acetone in a volume ratio of 100:1-1:1, and the fraction is analyzed by silica gel thin layer chromatography (TLC), The same fractions were combined to obtain 9 components (F1-F9); component F4 was subjected to reverse phase silica gel column chromatography, eluted with methanol-water with a volume ratio of 10%-100%, and the obtained fractions were prepared using Reversed-phase column (C 18 , 5μm, 10 × 150mm), separated by isocratic elution with methanol-water solution with a concentration of 40% to obtain the compound of formula (I) terpineol-8-O-...
Embodiment 3
[0043] A, get the aerial part 2kg of blue orchid plant Dracocephalum komarovi Lipsky, after pulverizing, use the dehydrated alcohol of 7 times of volume concentration to percolate and extract 3 times at room temperature, evaporate the solvent to dryness under reduced pressure to obtain extract;
[0044] b. The extract obtained in step a is separated by a normal-phase silica gel column, and the gradient elution is carried out with n-hexane-ethyl acetate in a volume ratio of 100:1-1:1, and the fraction is analyzed by silica gel thin layer chromatography (TLC) , the same fractions were combined to obtain 9 components (F1-F9); component F4 was separated by a Sephadex LH-20 gel column, and eluted with methanol-water with a volume ratio of 10%-100%. Preparative reversed-phase column (C 18 , 5 μm, 10 × 150 mm), separated by isocratic elution with methanol-water solution with a concentration of 40% to obtain the compound of formula (I) as terpineol-8-O-D-glucopyranosyl-(1→2)-β -D-glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com