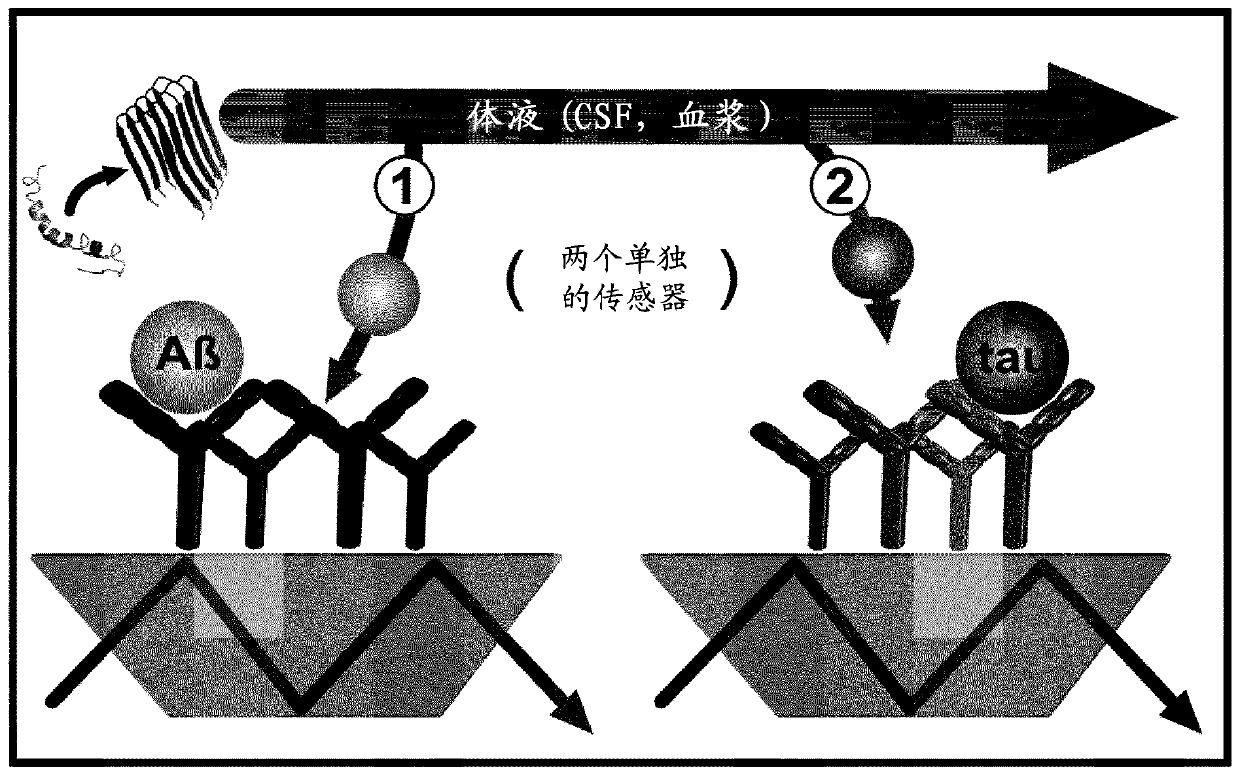
Combined assay for differential diagnosis of alzheimer's disease
A technology for Alzheimer's disease and differential diagnosis, applied in the field of combined immuno-infrared assays, which can solve the problem of no spectral decomposition, inability to reveal direct secondary structure changes of proteins, and inability to provide a robust platform for protein secondary structure transition analysis, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
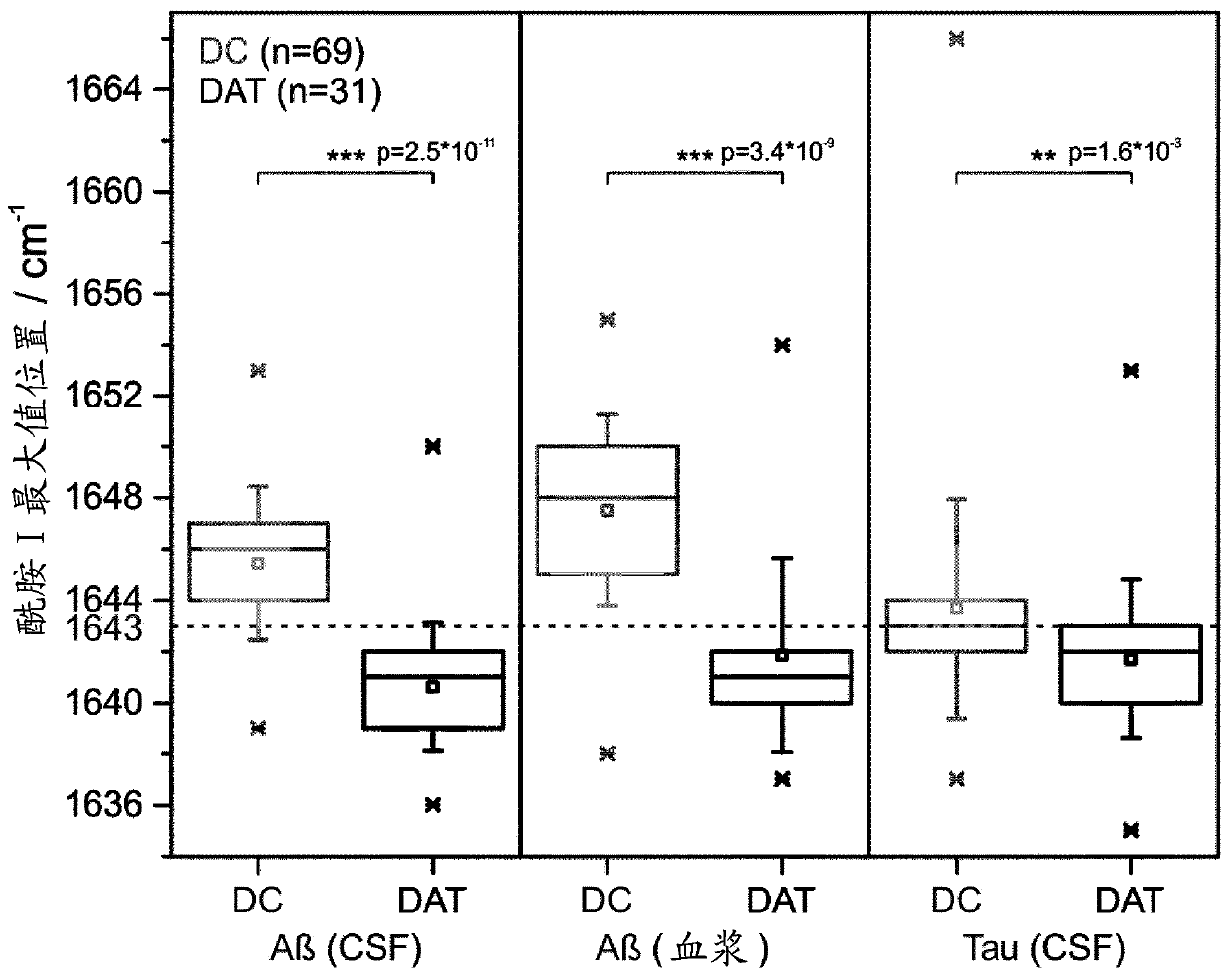
[0080] Example 1: Aβ and Tau protein secondary structure profiles for precise DC and DAT discrimination.
[0081] The performed study included 300 samples from 61 DC and 39 DAT patients. Details regarding the patient's differential diagnosis were described previously (Nabers et al., Anal. Chem. Doi: 10.1021 / acs.analchem.5b04286 (2016)). Typically, the patient set is divided into DC and DAT subjects. The DAT group was further subdivided into early, moderate, and severe stages of Alzheimer's disease. For a minority of DC patients, a full differential diagnosis is available, including those with dementias due to origins other than Alzheimer's disease, such as Parkinson's disease or vascular dementia. To analyze the secondary structure distribution of Aβ and Tau in CSF and / or plasma, the two biomarkers were extracted from the respective fluids by the immunoinfrared sensor described by Nabers et al. (Nabers et al., Anal. Chem. Doi: 10.1021 / acs.analchem.5b04286(2016)). Thus, ...
Embodiment 2
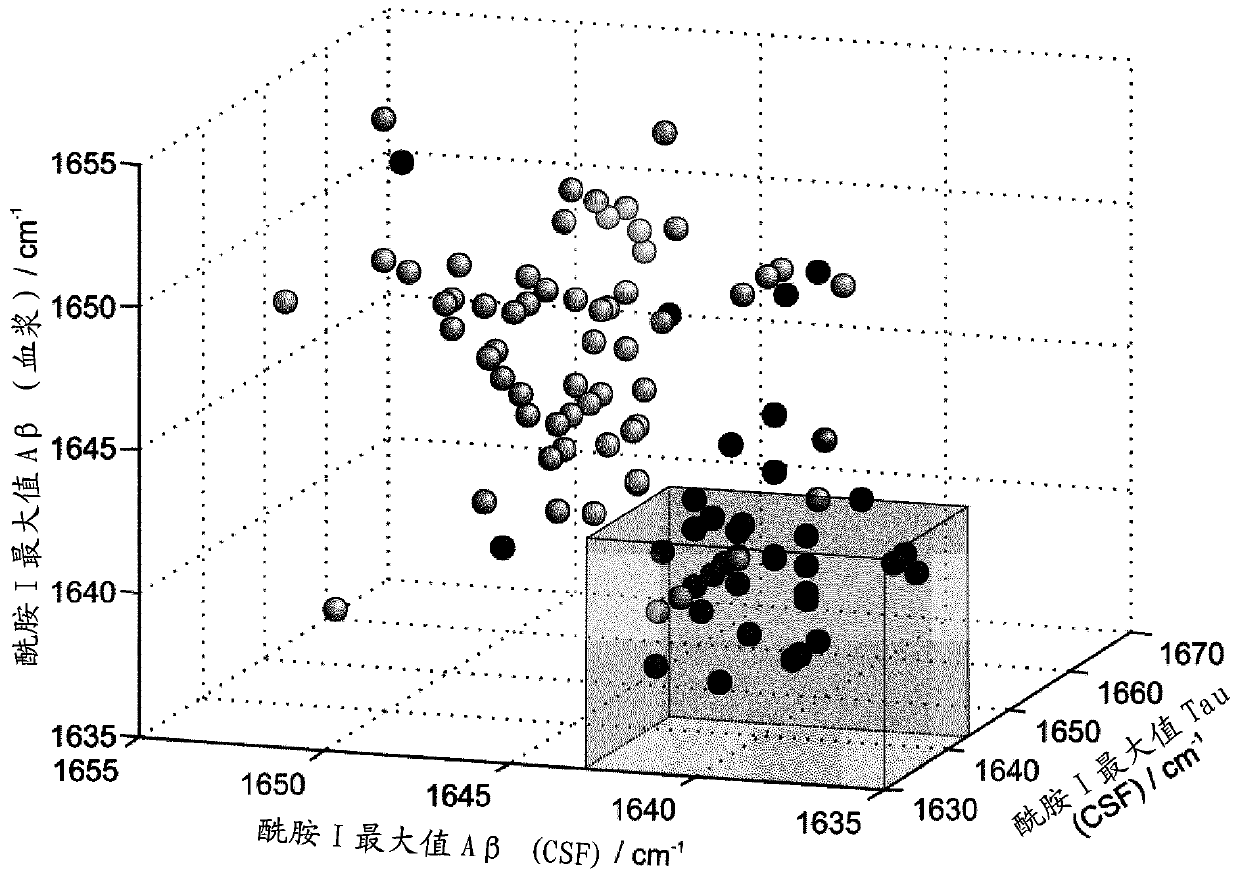
[0082] Example 2: Combination assay for differential diagnosis of DC and DAT.
[0083] Combined data analysis also offered the potential to subdivide the two diagnostic groups. this is in Figure 5 is schematically shown in . For example, Aβ from CSF and plasma showed an amide I maximum greater than or equal to 1643 cm -1 , but the maximum value of Tauamide I is lower than 1643cm -1 , in this case another type of dementia can be indicated by combined immuno-infrared assays ( Figure 5 ). Conversely, when Aβ amide I maxima from CSF and plasma are below the marker band but Tau maxima are above this, an early state of DAT will be indicated. This procedure was applied to both diagnostic groups in our study. Amide I maxima for Aβ from CSF demonstrated a higher maxima than Tau from CSF in 69% of all DC cases. This effect can be explained by the higher disordered nature of Tau protein compared to Aβ. On the other hand, the maximum value of Aβ was lower in 25% of all DC cases ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com