Composition for alleviating atopic skin symptoms, containing poly-gamma-glutamic acid
A technology for atopic dermatitis and glutamic acid, applied in skin care preparations, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to Embodiment 3 and comparative example 1 and comparative example 2
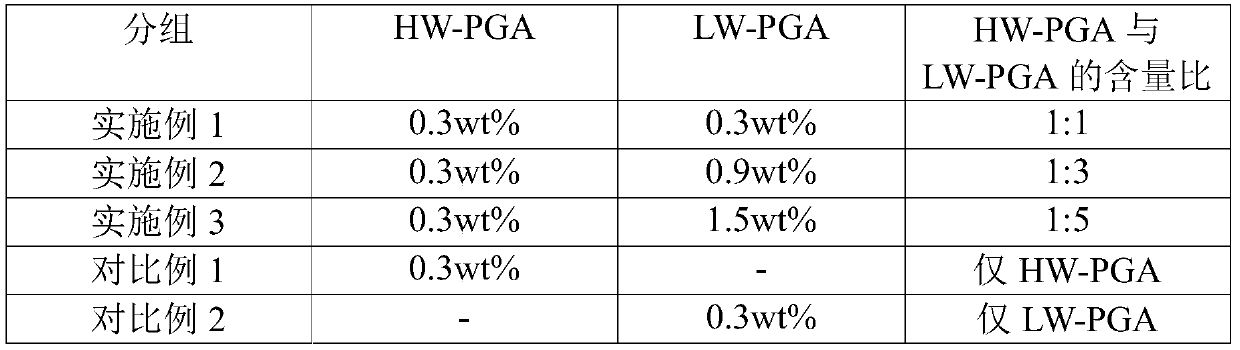
[0028] In the content ratio specified in Table 1 below, high-molecular-weight poly-γ-glutamic acid (2,000 kDa; hereinafter referred to as "HM-PGA") and low molecular weight poly-γ-glutamic acid (1kDa; hereinafter referred to as "LM-PGA"), to prepare 2 cream formulations.
[0029] Table 1
[0030]
experiment example 1
[0031] Experimental Example 1: Evaluation of the effect of formulations containing HM-PGA alone or LM-PGA alone or a combination of the two in improving symptoms of atopic dermatitis
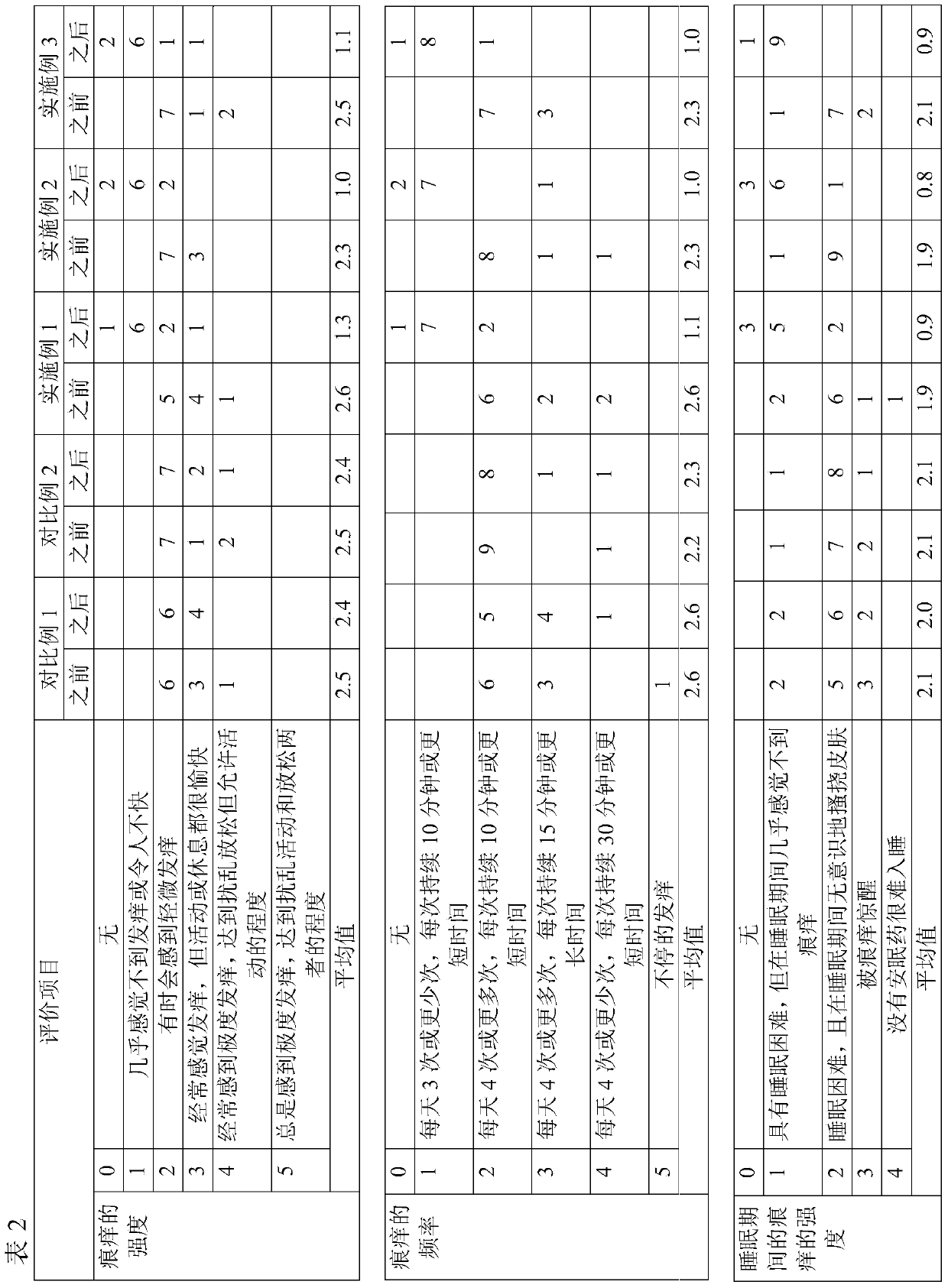
[0032] Each of the formulations of Examples 1 to 3 and Comparative Examples 1 and 2 were administered to 10 participants (male or female, different ages) suffering from symptoms of atopic dermatitis for 1 week. Each participant was asked to self-assess the intensity and frequency of itching before and after application of the formulation and the intensity of itching during sleep. The evaluation results are shown in Table 2 below (unit: person).
[0033] -Test object
[0034] (1) Gender: male (50%), female (50%)
[0035] (2) Age: 5 to 9 years old (50%), 10 to 15 years old (33%), 15 to 19 years old (17%)
[0036]
[0037] As can be seen from Table 2, the formulations of Comparative Example 1 and Comparative Example 2, which used one of the two active ingredients of the present invention alon...
experiment example 2
[0039] Experimental Example 2: Skin Patch Stability Test
[0040] The formulations of Examples 1 to 3 were evaluated for stability as skin patches.
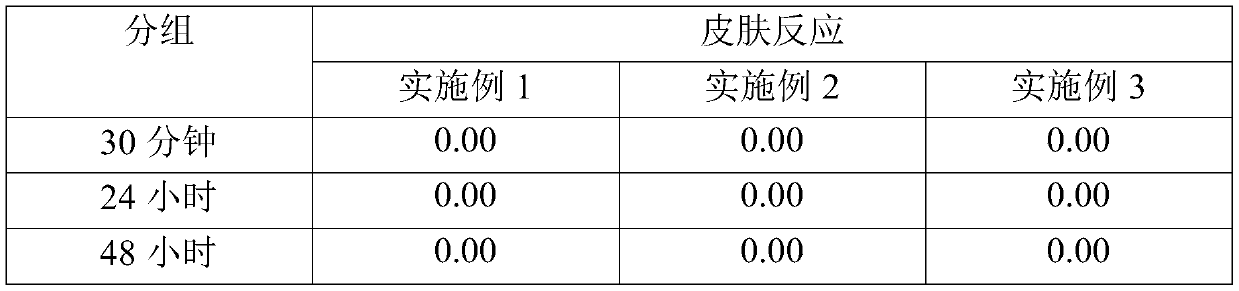
[0041] A skin patch trial was completed on 33 participants using the Finn Chamber. The back of each participant was washed with 70% ethanol and dried, and 20 μl of the test substance was added dropwise on an 8 mm diameter Finn Chamber. The patch is placed on the back and removed after 24 hours. Skin reactions were read at 30 minutes, 24 hours and 48 hours after application. The procedures were performed by dermatologists, and the assessment was performed according to the criteria of the International Contact Dermatitis Research Group (ICDRG). The evaluation results are shown in Table 3 below.
[0042] table 3
[0043]
[0044] As can be seen in Table 3, the composition of the present invention elicited a skin reaction of 0.00 at 30 minutes, 24 hours and 48 hours after removal of the patch and was considered "non-irritatin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com