Tau-protein targeting protacs and associated methods of use
A technique for selecting, chemical structures, applied in the field of bifunctional compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
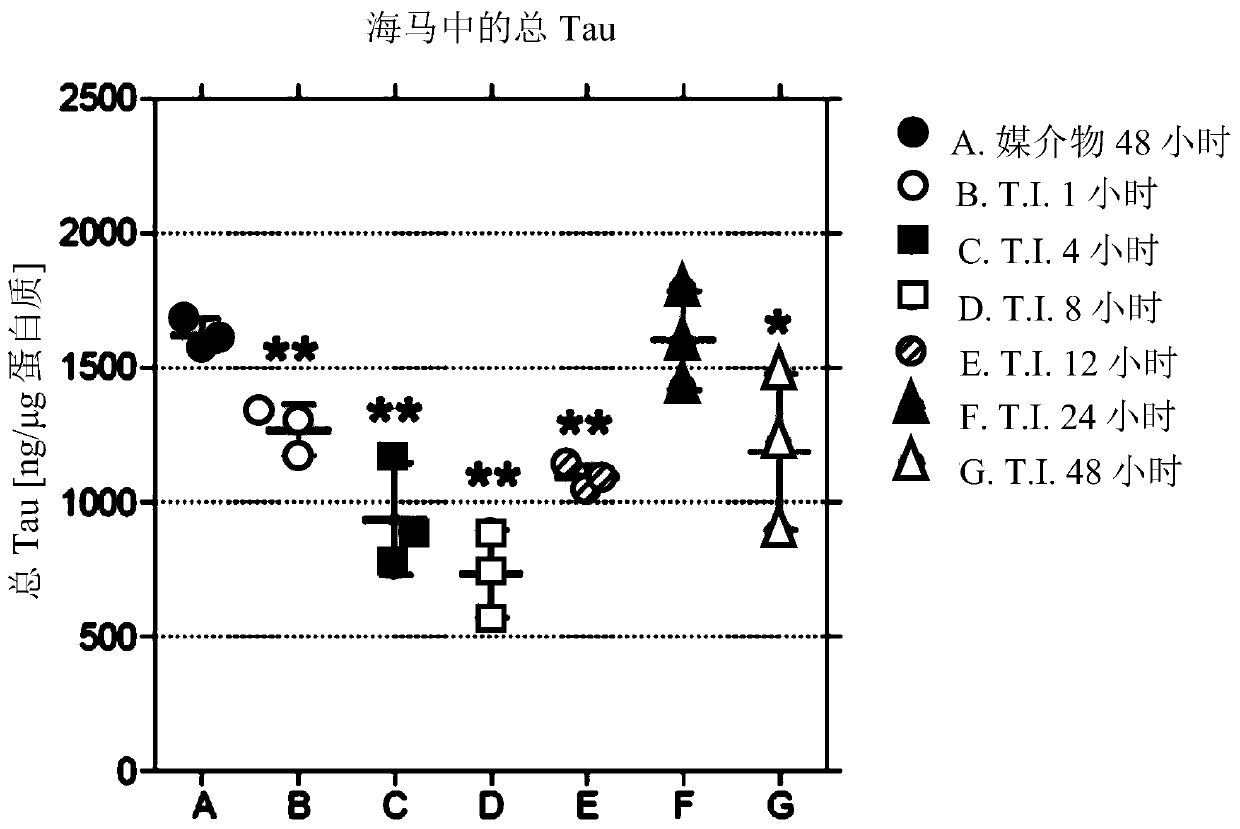
[0761] PROTAC compounds of the present disclosure are effective in Tau degradation. Exemplary compounds are presented in Tables 1 and 2, with in vitro data for some selected compounds in Tables 2 and 3 showing degradation of tau protein. In vivo studies showing tau protein degradation in figure 1 shown in .
[0762] General Methods of Chemical Synthesis
[0763]Synthesis of the claimed chimeric compounds can be carried out according to general synthetic procedures known in the literature. The synthetic routes shown in the schemes in this disclosure are described as one of the methods that can be used to obtain the desired compounds. Other methods may also be useful to those skilled in the synthetic arts. The ULM and PTM described in the scheme represent only one of many ULMs and PTMs in this patent application.
[0764] LC-MS Method for Purity Analysis (Quality Control)
[0765] LCMS method:
[0766] Instruments: Agilent infinity 1260 LC; Agilent 6230 TOF mass spectr...
specific Embodiment
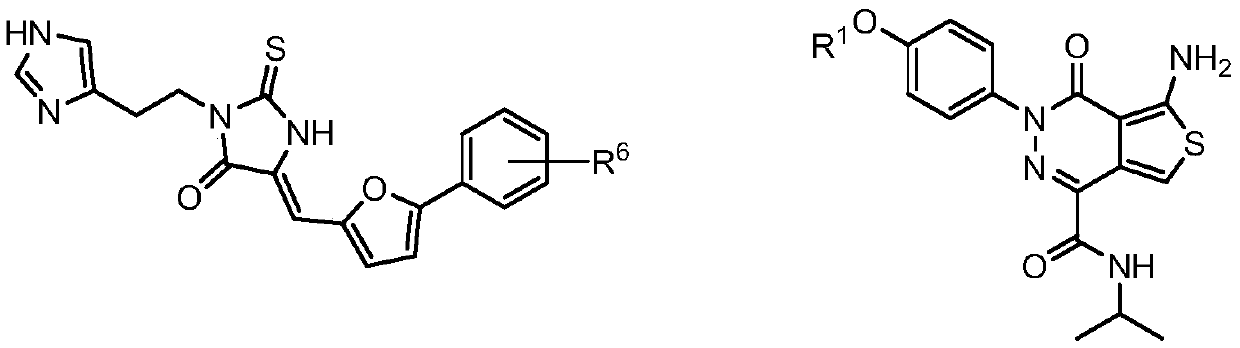
[2376] One aspect of the present disclosure provides bifunctional compounds having the following chemical structures:
[2377] ULM-L-PTM,
[2378] or a pharmaceutically acceptable salt, enantiomer, stereoisomer, solvate, polymorph or prodrug thereof,
[2379] in:
[2380] The ULM is a small molecule E3 ubiquitin ligase binding moiety that binds E3 ubiquitin ligase;
[2381] The PTM is a Tau protein targeting moiety; and
[2382] Said L is a bond or a chemical linkage moiety linking ULM and PTM.
[2383] In any aspect or embodiment described herein, the E3 ubiquitin ligase binding moiety targets an E3 ubiquitin ligase selected from VonHippel-Lindau (VLM) and cereblon (CLM).
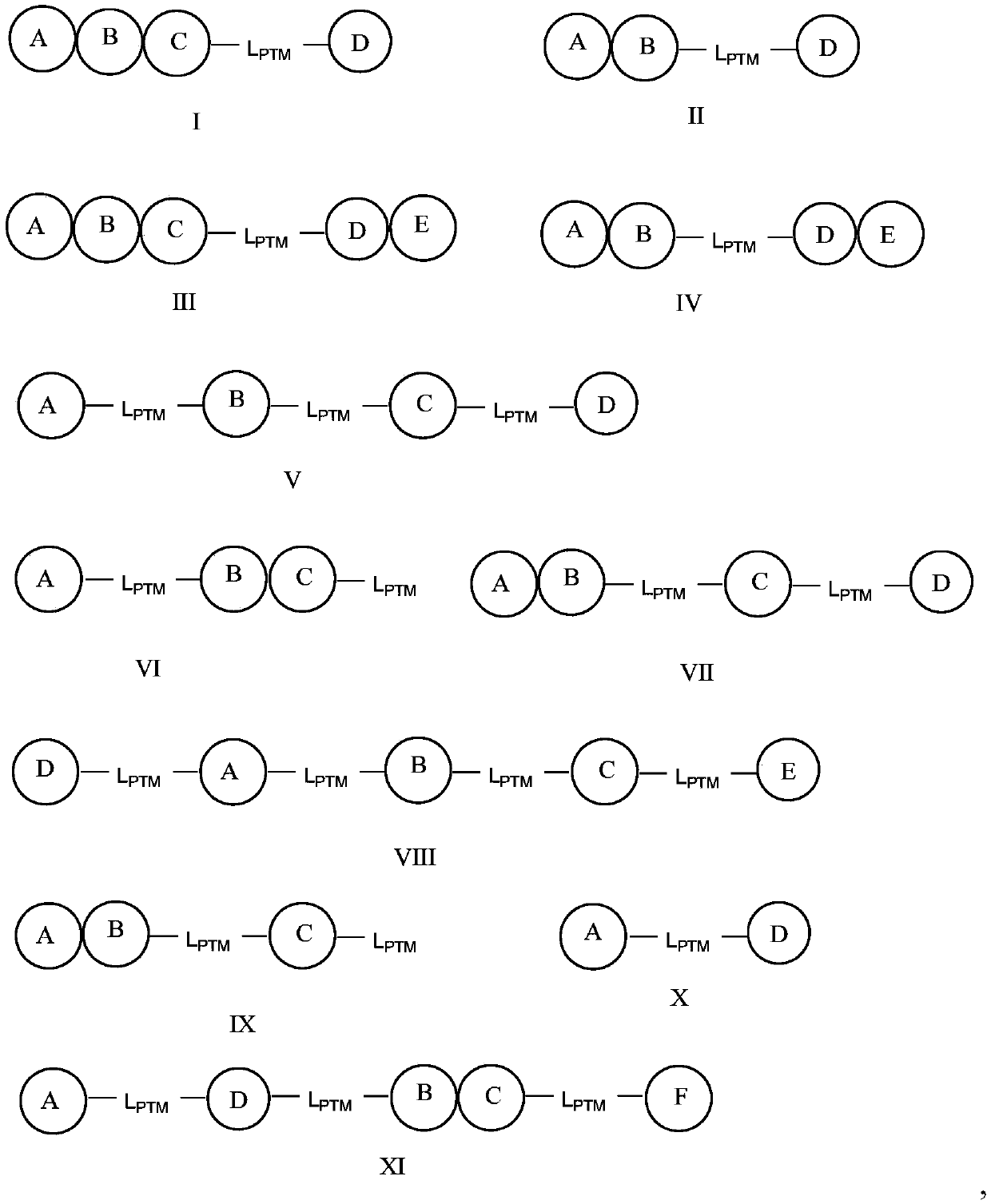
[2384] In any aspect or embodiment described herein, the PTM is represented by Formula I, II, III, IV, V, VI, VII, VIII, IX, X or XI:
[2385]
[2386] in:
[2387] A, B, C, D, E and F are independently selected from an optionally substituted 5 or 6 membered aryl or heteroaryl ring, an optionally ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com