Fusion protein of IL-2 mutant and antibody and application of fusion protein
A fusion protein, mutant technology, applied in the field of biotechnology and antibody engineering, can solve problems such as low reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Example 1: Treg cells inhibit WT IL-2 from exerting its biological function
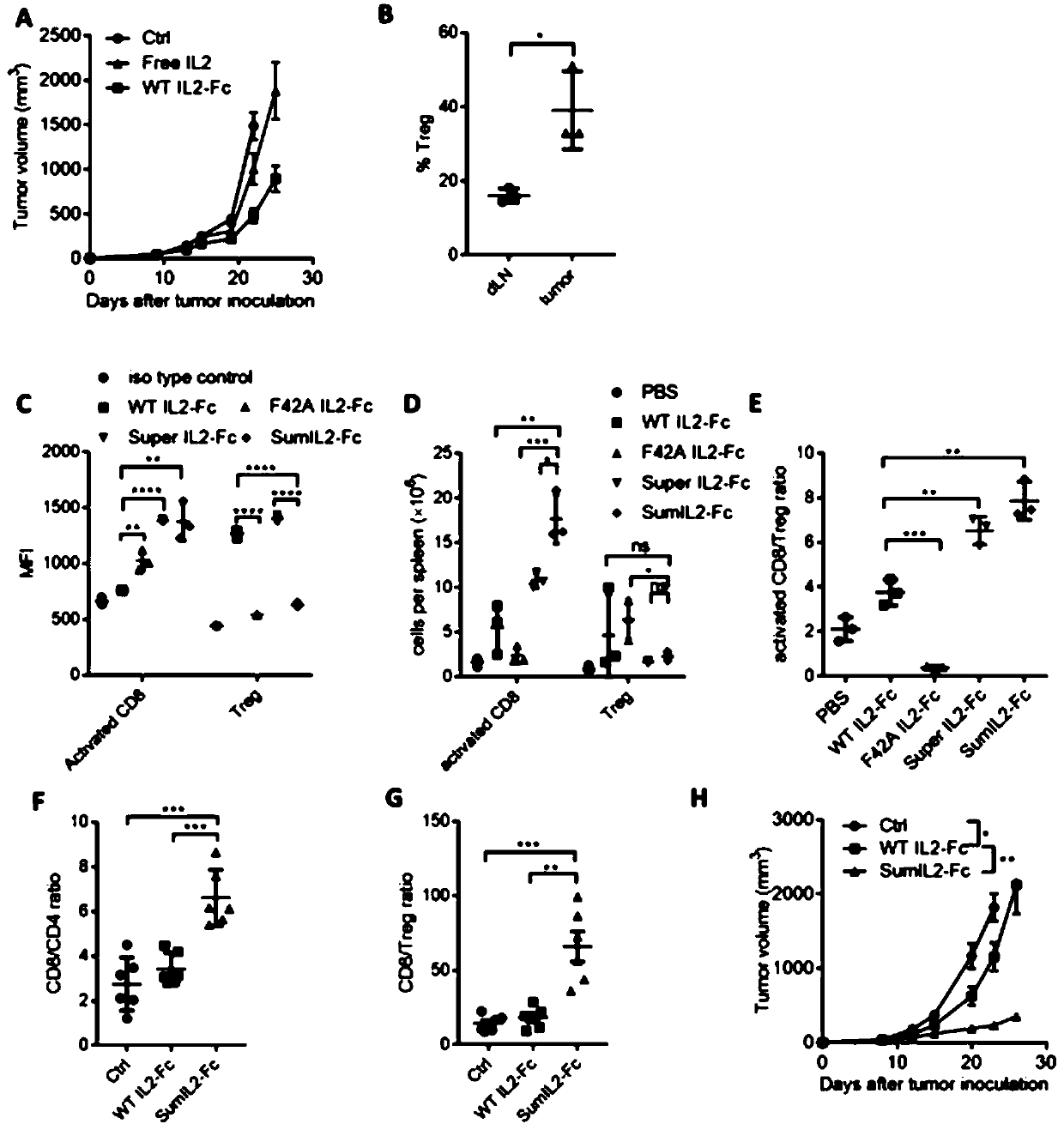
[0143] Considering the toxic and side effects of clinical use of large doses of IL-2, we fused IL-2 with the Fc of human antibody IgG1, and expressed and purified it using a mammalian suspension cell expression system. Low doses of Free IL-2 could not control the growth of tumors. When B16F10 tumor-bearing mice were treated with the same dose of IL2-Fc, the tumor growth of mice was relatively controlled but still showed an upward trend ( figure 1 A). This result indicated that the wild-type IL-2 also played the function of suppressing the immune response in the process of functioning. Through detection, it was found that there were more Tregs in tumor tissue than in lymphoid organs ( figure 1 B). This suggests the necessity of reducing IL-2 binding and inducing activation of proliferating Tregs.
Embodiment 2
[0144] Example 2: sumIL-2 enhances the binding and expansion of activated CD8 T cells, reduces the binding and expansion of Treg cells
[0145] We introduced the above six mutation sites in IL-2. We expressed and purified the fusion Fc forms of WTIL-2-Fc and sumIL-2-Fc, and tested the ability of the two fusion proteins to bind to the receptor and bind to effector cells in vitro.
[0146] To characterize the ability of WT IL-2 and sumIL-2 to bind different T cell subsets, we compared the differences in T cell binding of IL-2 fusion proteins by in vitro cell binding assays. In the same spleen cells,
[0147] (1) The ability of WT IL-2 to bind Treg cells is higher than that of memory CD8+ T cells ( figure 1 C);
[0148] (2) On the contrary, sumIL-2 is more inclined to bind memory CD8 + T cells but not Treg cells ( figure 1 C).
[0149] These in vitro experiments demonstrate that sumIL-2 is designed to activate memory CD8+ T cells rather than Treg cells.
[0150] In order...
Embodiment 3
[0151] Example 3: SumIL-2 has stronger anti-tumor activity than WT IL-2
[0152] In order to compare the anti-tumor function of the constructed IL-2 mutant and wild-type IL-2 in vivo, we used the mouse melanoma model to evaluate. SumIL-2 showed a tendency to bind activated CD8+ T cells in in vitro cell binding experiments, so the changes in T cells in tumors after treatment with sumIL-2 or WT IL-2 were first detected.
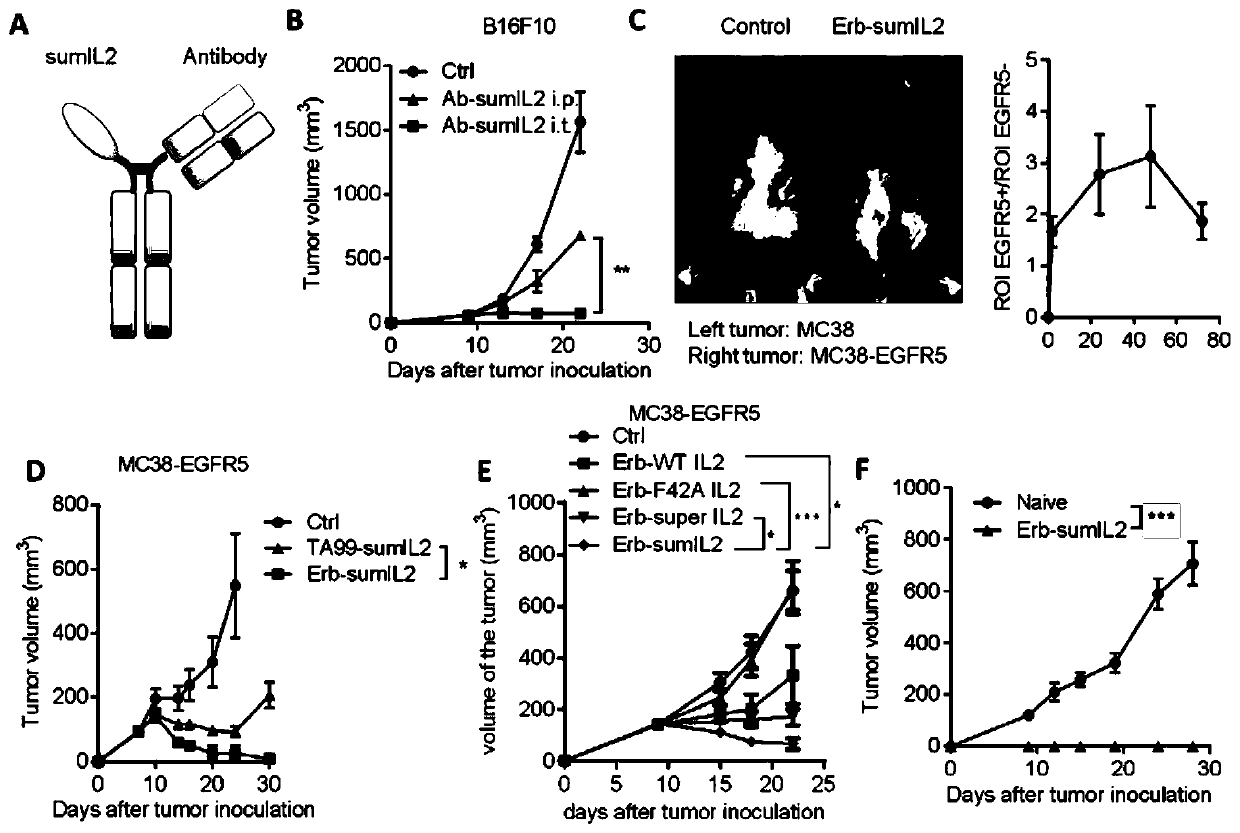
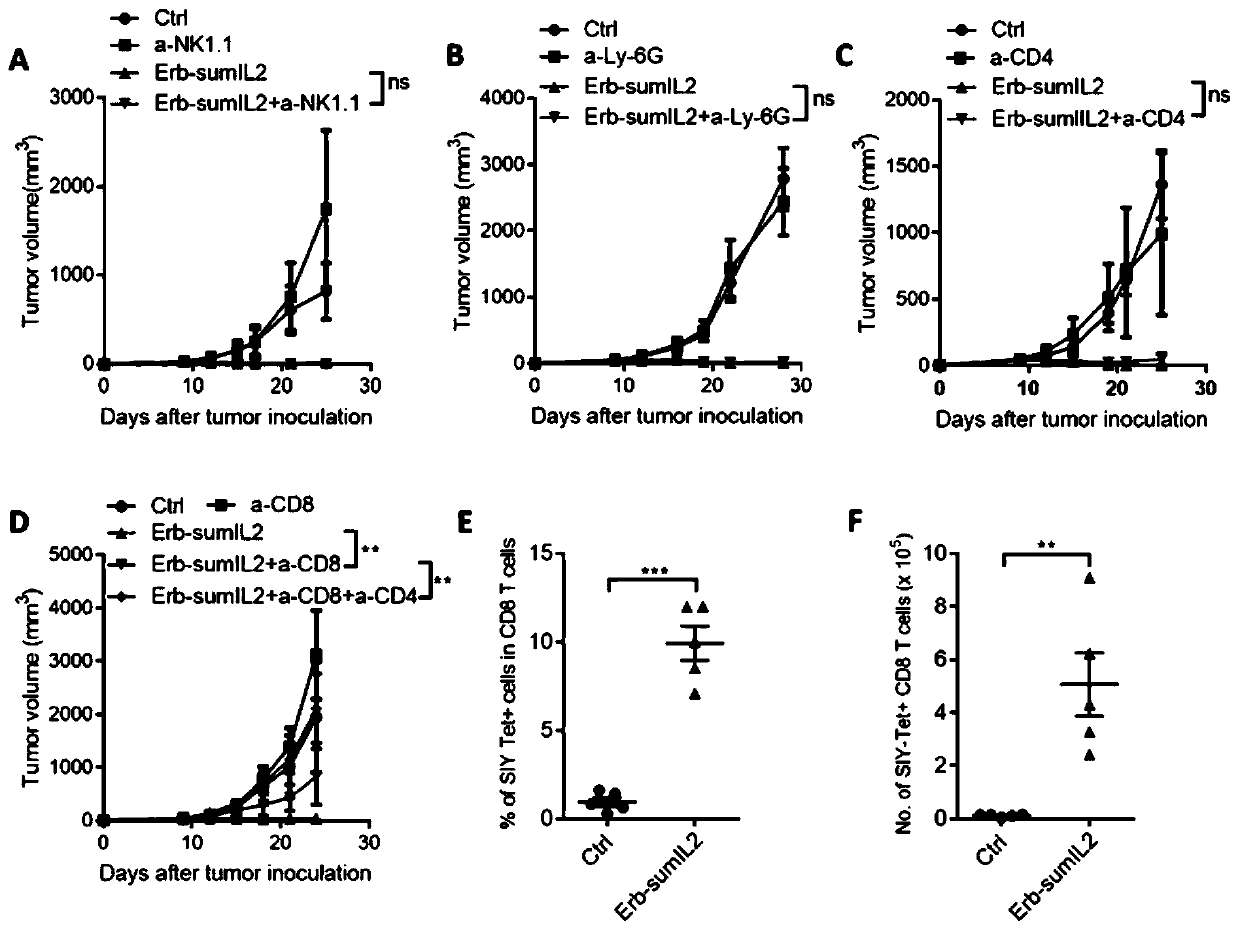
[0153] The same dose (5 μg) of sumIL-2-Fc and WT IL-2-Fc fusion protein was injected into the tumor, and the change of the cell ratio in the mouse tumor was detected by flow cytometry 3 days later. CTL is a very important factor associated with tumor regression, we found that sumIL-2 can increase the ratio of CD8 / CD4 ( figure 1 F), and can increase the ratio of CD8 / Treg ( figure 1 G), which indicates that mutant IL-2 can indeed expand more CD8+ T cells in the tumor. With such strong evidence, we further evaluated the tumor therapeutic effects of sumIL-2 an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com