Method for producing gallium nitride crystal
A manufacturing method and gallium nitride technology, applied in chemical instruments and methods, crystal growth, single crystal growth, etc., can solve problems such as ignition or explosion, safety problems, and sodium vapor leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
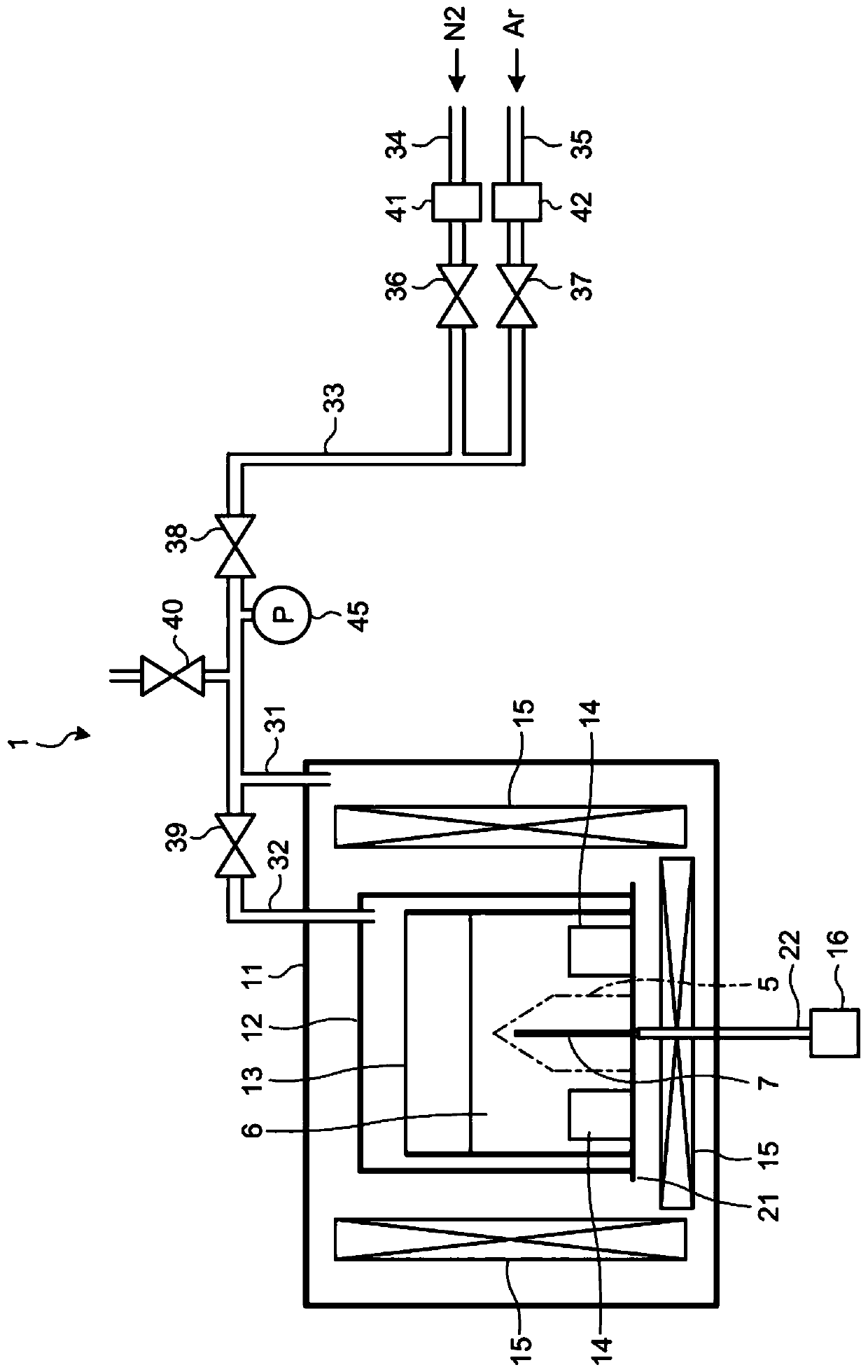
[0029] Embodiments of a method for manufacturing gallium nitride crystals will be described in detail below with reference to the accompanying drawings. figure 1 is a diagram illustrating an example of the configuration of the manufacturing apparatus 1 used in the manufacturing method of the gallium nitride crystal according to the first embodiment. Manufacturing apparatus 1 is an apparatus that manufactures gallium nitride crystal 5 by using a flux method.
[0030] The pressure-resistant container 11 is made of, for example, stainless steel. Inside the pressure-resistant container 11, an inner container 12 is provided. Inside the inner container 12, a reaction container 13 is accommodated.
[0031] Reaction vessel 13 is a vessel for storing Ga—Na mixed melt (flux) 6 and seed crystal 7 and for growing gallium nitride crystal 5 . The material of the reaction vessel 13 is not limited to any specific material. Examples of the material of the reaction vessel 13 may include nit...
no. 1 example
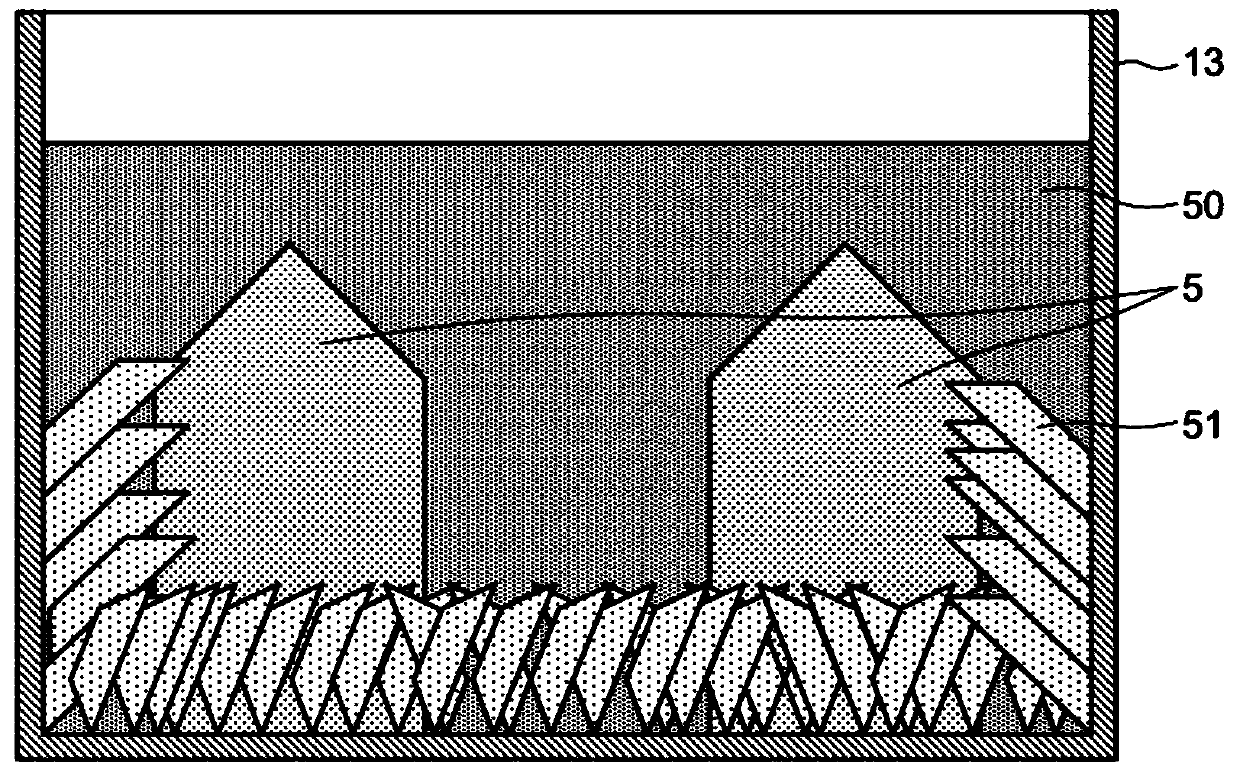
[0067] The first embodiment is described below. In the first example, gallium nitride crystal 5 was produced using production apparatus 1 according to the first embodiment. After the crystal growth is completed, the gallium 55 is separated from the alloy 51 remaining in the reaction vessel 13, and the gallium 55 is collected therefrom.
[0068] crystal growth
[0069] In a reaction vessel 13 made of alumina housed in a glove box having a high-purity argon atmosphere therein, a gallium nitride (GaN ) crystals consisting of two seeds 7 . The seed crystal 7 was inserted into a hole made on the bottom surface of the reaction vessel 13 to a depth of 4 mm.
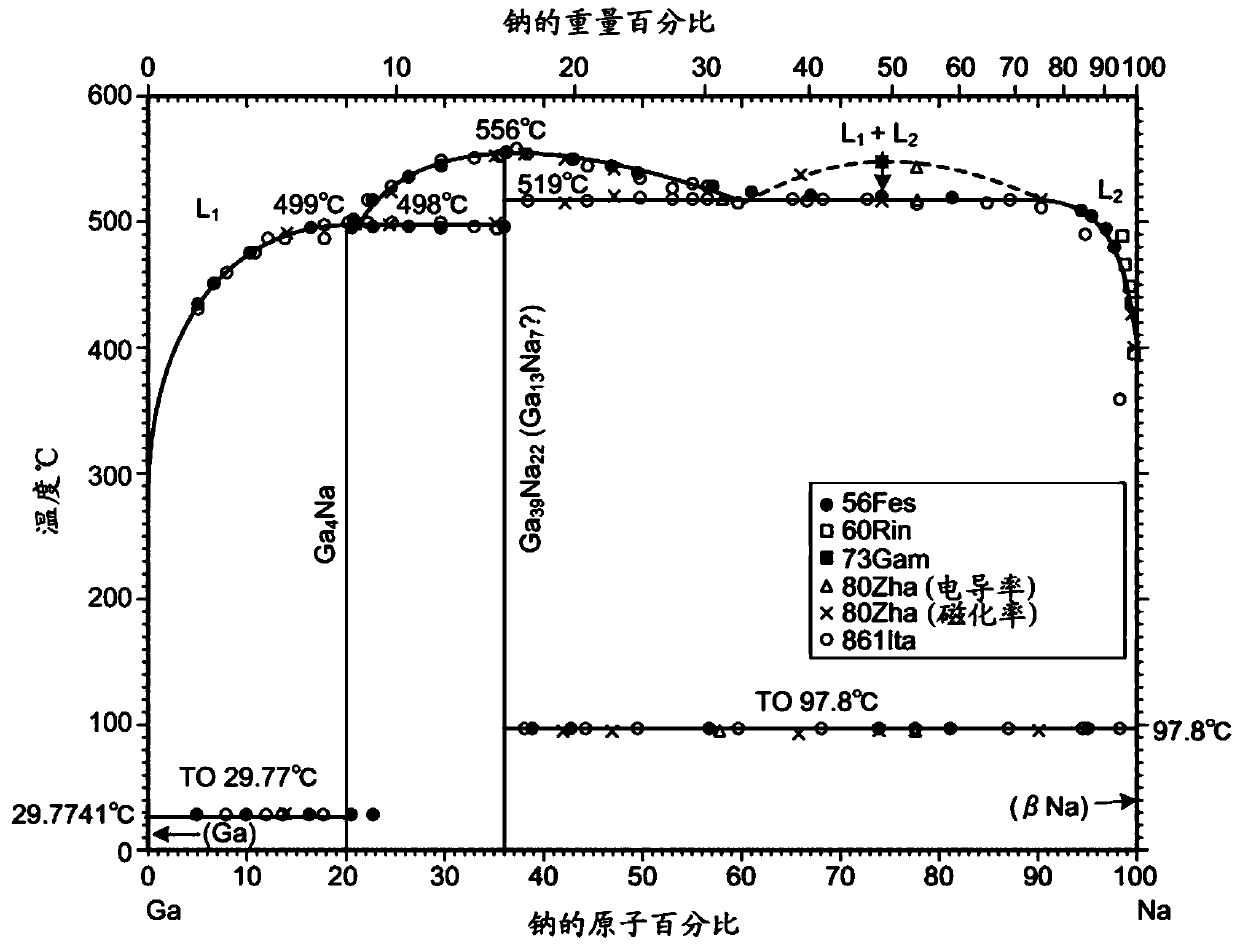
[0070] Subsequently, heated and liquefied sodium (Na) is poured into the reaction vessel 13 as a mixed melt 6 . After the sodium solidifies, gallium (Ga) and carbon are added. The molar ratio of gallium to sodium was set at 0.25:0.75. The amount of carbon added to the mixed melt 6 was 0.5 mol % relative to the total molar ...
no. 2 example
[0082] In the second embodiment, the same processes as in the "crystal growth" and "removal of sodium" steps in the first embodiment are performed, and then gallium 55 is collected using acid.
[0083] Gallium Separation and Collection
[0084] Gallium nitride crystal 5 and alloy 51 remain in reaction vessel 13 after sodium 50 is removed. All the ethanol in the stainless steel container 54 was drained, and then the water 52 was poured into the container 54 . The container 54 was placed on a hot plate 56 and the temperature of the hot plate 56 was adjusted to bring the temperature of the water 52 in the container 54 to about 70°C. The water 52 reacts with the sodium in the alloy 51 and produces hydrogen gas 53 . The gallium 55 detached from the alloy 51 was aspirated through the pipette 57 several times during the reaction. After 1 hour had elapsed since the water 52 had been poured into the reaction vessel 13, the alloy 51 remaining in the reaction vessel 13 was transferred...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com